PRODUCT IDENTIFICATION
5144-89-8 (Monohydrate)
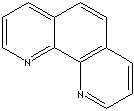
EINECS NO.
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
Phenanthrene is a tricyclic aromatic hydrocarbon composed of three fused benzene rings (isomeric with anthracene) occurring in coal tar and produced by the degradation of certain alkaloids. It is a white crystalline solid; melt point 117 C. It is toxic and carcinogenic. It is used as a starting material to prepare dyes and drugs. Phenanthroline is any of three nitrogen bases which two carbon atoms are replaced by two nitrogens in the ring structure. Phenanthrene has five resonance structures. There are three isomeric phenanthrolines of which names indicate nitrogen positions; 1,7- ,1,10- , and 4,7-. Phenanthroline is a metal chelator. It is used in metallocene industry for the application including;
- Coordination of organometallic-complexes
- Redox mediators in biosensor
- Catalysts for the oxidative organic synthesis
- Molecular chemistry
- Disease diagnosis and treatment
- Water treatment
- Photolysis chemistry
- Microbiology
1,10-Phenanthroline (also called o-phenanthroline) used as an oxidation-reduction indicator due to characteristic color changes; turning faint blue when oxidized. It is used as an indicator to determine iron.
Related Categories:
- Chelating agent
- Cholinergic agent
- Cholinesterase inhibitor
- Cross-Linking Reagent
- Indicator
- Intercalating agent
- Neurotransmitter Agent
|
|
|
|
|
|
APPEARANCE
99.0% min
LOSS ON DRYING
1.0% max
RESIDUE ON IGNITION
0.1% max
2811