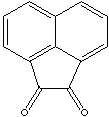
| ACENAPHTHENEQUINONE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 82-86-0 |
|
| EINECS NO. |
201-441-3 | |
| FORMULA | C10H6(CO)2 | |
| MOL WT. | 182.18 | |
| H.S. CODE | ||
| TOXICITY | ||
| SYNONYMS | 1,2-Diketoacenaphthene; Acenaphthenequinone; | |
| 1,2-Acenaphthylenedione; Acenaphthoquinone; 1,2-Acenaphthenequinone; Acenaphthylene-1,2-quinone; | ||
| SMILES | coal tar | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | yellow crystals | |
| MELTING POINT |
257 - 259 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Insoluble (soluble in alcohol) | |
|
SOLVENT SOLUBILITY |
||
| AUTOIGNITION | ||
| pH | ||
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 2; Flammability: 1; Reactivity: 1 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
Diketone is a molecule
which contains two ketone carbonyl groups. Diacetyl (CH3COCOCH3),
2,3-butadione, is the simplest aliphatic diketone.
It is an alpha-diketone which has two ketone groups side-by-side.
Usually, alpha-diketone imparts a carmel like or buttery flavor.
Diketone compounds take a role in creating various
fragrances. Bezil (C6H5COCOC6H5)
is
an aromatic diketone, the fundamental structure of
photo sensitive molecule which is broken down into free radicals upon exposure to ultraviolet
radiation. Acetoacetone
is a beta-diketone which two ketones are separated only by one carbon.
The beta-ketone is stable as a conjugated enol rather than a diketone
due to the delocalization which makes the counterion more stable and less likely to regain the
proton. Ascorbic acid is an example of enol compound. Enol compounds form complexes with many transition metal
ions. These compounds are readily soluble in organic solvents. They are widely used as chelating
agents, ligands, and catalyst precursors.
Acetoacetic acid and its esters contain active
methylene groups which have relatively acidic alpha-protons due to H atom
adjacent to two carbonyl groups. The reactivity of its methylene group provide
the sequence of reactions of alkylation, hydrolysis of the esters and
decarboxylation resulting in substituted ketones. Acetoacetic acid derivatives
are important aliphatic parts adjoining azo dyes and pigments.
Aacetoacetate
is one of Ketone bodies which are the
end-products of rapid or excessive fatty acid breakdown in the human body. Para-benzoquinone
and its derivatives belong to 1,4-diketone family. Benzoquinone is used as an oxidizing agent
in organic chemistry and is a common constituent of biologically molecules like
Vitamin K1. Quinones serves as electron acceptors in electron transport chains such as in
photosynthesis, and aerobic
respiration. Diketene
derivatives find versatile applications in making biomolecules, agrochemicals, dyes, pigments,
pharmaceuticals including vitamins, and stabilizers for PVC and
polyester. They are used as components for fragrances and as solvents. Diketones
undergo the reversible and irreversible addition reactions include;
1,2-acenaphthylenedione (tricyclic) unsaturated hydrocarbon derived from naphthalene derivative, is used as an intermediate for dyes, pharmaceuticals, pesticides. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow crystals | |
| ASSAY (GLC) |
97.0% min | |
| MELTING POINT |
91 C min | |
|
ASH |
0.2% max | |
| MOISTURE (K.F) | 0.1% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in drum | |
| HAZARD CLASS | ||
| UN NO. |
| |
OTHER INFORMATION |
||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 26-37/39 | ||