PRODUCT IDENTIFICATION
UN NO.
CLASSIFICATION
Piperidine, Light stabilizer
PHYSICAL AND CHEMICAL PROPERTIES
0.84
REFRACTIVE INDEX
124 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Local:
Piperidine, hexahydropyridine, is a family of
heterocyclic organic compound derived from pyridine through
hydrogenation. It has one nitrogen atom in the cycle. It is a
clear liquid with pepper-like aroma. It boils at 106
C, soluble in water, alcohol, and ether. The major application of piperidine is
for the production of Dipiperidinyl dithium tetrasulfide used as a rubber
vulcanization accelerator. In pharmaceutical synthesis industry, it is used as a
special solvent and a protecting group for peptide synthesis.
Piperidine derivative compounds are used as intermediate to make
crystal derivative of aromatic nitrogen compounds
containing nuclear halogen atoms. Ring system compounds with nitrogen which have
basic property play important roles as cyclic component in industrial field such
as raw materials for hardener of epoxy resins, corrosion inhibitors,
insecticides, accelerators for rubber, urethane catalysts, antioxidants and as a
catalyst for silicone esters. They are used in manufacturing
pharmaceuticals.
2-Piperidone, a keto-hydropyridine, is a 6-membered lactam structure compound (delta-valerolactam). Lactam structure compounds including delta-valerolactam are good organic solvents soluble in water. Cyclic amides and analogues including N-alkyl lactams are biologically active nucleuses in pharmacologicals and flavorings. Lactams are widely used in artificial fibre industry. They are polymerizable and used as nylon precursors. They have excellent applications as solvents and as intermediates for organic synthesis. They are used in the aqueous carrier medium and amide penetrants in inks and water soluble paints. 4-Piperidone, a cyclic amide with ketone at para position, and its N-alkyl substituted derivatives are used in:
- Alkene formation at the position of former carbonyl group. Alkene bonds are abundant in natural compounds
- Sulfur compound removals
- UV stabilizer products
- Multicyclic hetero compounds and fused ring system compounds
- Pharmaceutical synthesis including narcotic analgesics and digestive system drugs
2,2,6,6-Tetramethylpiperidinone, cyclic amide containing ketone at 4 position and polymethyl branches, is the parent compound of many hindered amine light stabilizer (HALS) and other organic chemicals. HALS acts as a catalyst to minimize the activation of the peroxy radicals to prevent discoloring and staining in varnishes, rubbers and polymers.
Amidines ( -HN=CNH2) and guanidines [-HN=C(NH2)2] are strongly basic reagents. Bases activation of deprotonation is the first step in the synthesis. The strength of basicity, nucleophilicity, steric hindrance or solubility are the factors to select reagents for the best results in the chemical reactions such as alkylation, tautomerization, dehydrohalogenation, michael-addition, esterification, saponification, acylation, silylations, aldol-condensation, eliminations, and cyclization. The order of basicity strength of some amines are; TBD > MTBD > DBU > DBN > TMG > Quinuclidine > TMP, Pempidine > Triethylamine > TED > Tributylamine > Collidine > Lutidine
- TBD: 1,5,7-Triazabicyclo(4.4.0)dec-5-ene; 1,3,4,6,7,8-Hexahydro-2H-pyrimido[1,2-a] pyrimidine, CAS #: 5807-14-7
- MTBD :7-Methyl-1,5,7-triazabicyclo(4.4.0)dec-5-ene;1,3,4,6,7,8-Hexahydro-1- methyl-2H-pyrimido [1,2-a]pyrimidine, CAS #: 84030-20-6
- DBU: 1,8-Diazabicyclo[5.4.0]undec-7-ene; 2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a] azepine, CAS #: 6674-22-2
- DBN: 1,5-Diazabicyclo[4.3.0]non-5-ene, CAS #: 3001-72-7
- TMG: 1,1,3,3-Tetramethylguanidine; N,N,N',N'-Tetramethylguanidine, CAS #: 80-70-6
- Quinuclidine: 1-Azabicyclo[2.2.2]octane, CAS #: 100-76-5
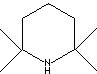
- TMP: 2,2,6,6-Tetramethylpiperidine, CAS #: 768-66-1
- Pempidine: 1,2,2,6,6-Pentamethylpiperidine, CAS #: 79-55-0
- TED: 1,4-Diazabicyclo[2.2.2]octan; Triethylenediamine, CAS #: 280-57-9
- Collidine: Trimethylpyridine, CAS #: 108-75-8 (2,4,6- Isomer)
- Lutidine: Dimethylpyridine, CAS #: 108-48-5 (2,6- Isomer)
APPEARANCE
98.0% min
1.0% max
PRICE INFORMATION