PRODUCT IDENTIFICATION
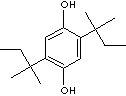
250.38
UN NO.
CLASSIFICATION
Antioxidant,
EXTRA NOTES
Primary antioxidant for organic polymers
PHYSICAL AND CHEMICAL PROPERTIES
1.05
Slightly soluble
Soluble in alcohol and benzene.
REFRACTIVE INDEX
124 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia
Linking
Antioxidants are frequently added to industrial products. A common use is as
stabilizers in fuels and lubricants to prevent oxidation, and in gasolines to
prevent the polymerization that leads to the formation of engine-fouling
residues.[243] In 2007, the
worldwide market for industrial antioxidants had a total volume of around 0.88
million tons. This created a revenue of circa 3.7 billion US-dollars (2.4
billion Euros). They are widely used to prevent the oxidative degradation of polymers such as rubbers, plastics and adhesives that causes a loss of strength and
flexibility in these materials.[245] Polymers
containing double bonds in their main chains, such as such as
natural rubber and polybutadiene, are especially
susceptible to oxidation and ozonolysis. They can be protected by antiozonants. Solid polymer
products start to crack on exposed surfaces as the material degrades and the
chains break. The mode of cracking varies between oxygen and ozone attack, the former causing a "crazy paving" effect,
while ozone attack produces deeper cracks aligned at right angles to the tensile
strain in the product. Oxidation and UV degradation are also frequently linked,
mainly because UV radiation creates free radicals by bond
breakage. The free radicals then react with oxygen to produce peroxy radicals which
cause yet further damage, often in a chain reaction. Other polymers susceptible to
oxidation include polypropylene and polyethylene. The former is more sensitive owing
to the presence of secondary carbon atoms present in every
repeat unit. Attack occurs at this point because the free radical formed is more
stable than one formed on a primary carbon atom. Oxidation of
polyethylene tends to occur at weak links in the chain, such as branch points in
low density polyethylene.
Material Safety Data Sheet (SongWon)
APPEARANCE
98.0% min
MELTING POINT
178 C min
VOLATILE LOSS
0.3% max
COLOR OF SOLUTION
97% (500nm)
0.1% max