PRODUCT IDENTIFICATION
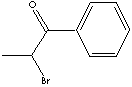
SMILES
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
clear to yellow liquid
REFRACTIVE INDEX
APPLICATIONS
Phenylethyl is a structural skeleton found in sympathomimetic amines that
have important physiological functions within the body as neurotransmitters in
the central nervous system and hormones in the blood circulation. Phenyl ketone
structure is found in keto alkaloids such as cathinone, a contributor of
stimulant effect. Phenyl ketone is a fuel in the brain. Propiophenone might be
an useful intermediate to prepare nervous system drugs (anxiolytic and hypnotic drugs).
Bupropion
is an example which has propiophenone moiety.
It is a constituent of synthetic perfumes, flavouring agents, and paints to stabilize other
ingredients. Ketones can be made by the oxidation of secondary alcohols and the destructive
distillation of certain salts of organic acids. In addition to as polar
solvents, ketones are important intermediates in the syntheses of organic
compounds such as alkoxides, hydroxyalkynes, imines, alcohols (primary,
secondary as well as tertiary), acetals, thioacetals, phosphine oxides, geminal
diols, hydrazones, organic sulfite and cyanohydrins.
APPEARANCE
clear to yellow liquid
95.0% min