PRODUCT IDENTIFICATION
CLASSIFICATION
AROMATIC AMINES / NITRO COMPOUNDS / DYES /
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
100 C
APPLICATIONS
- Michael addition
- Reduction
- Henry Reaction (Nitro-aldol reaction)
- Nef reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion reaction
- Alkylation, Acylation, and Halogenation
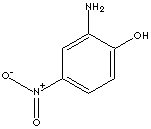
One of the most important aromatic amines is aniline; pale brown liquid boiling at 184 C, melting at -6 C. Aniline is obtained commercially from chlorobenzene by heating with ammonia in the presence of copper catalyst or from a product of coal tar (nitrobenzene) through the reduction reaction. Aniline is the starting material in the dye manufacturing industry and as in the manufacture of others. Aniline is converted into sulfanilic acid which is the parent compound of the sulfa drugs. Aniline is also important in the manufacture of rubber-processing chemicals, antioxidants and varnishes. Nitroanilines are used in the synthesis of dyes, agrochemicals, pharmaceuticals, rubber and plastic additives, photographic antifogging agents and coccidiostats. 2-Hydroxy-5-nitroaniline is used as an intermediate for pharmaceuticals, dyes 9especially reactive dyes), and other organic chemicals
APPEARANCE
98.0% min
138 C