PRODUCT IDENTIFICATION
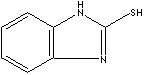
2933.90
Oral rat LD50: 300 mg/kg
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
301 - 305 C
1.42
REFRACTIVE INDEX
Antioxidant is a substance added in small quantities to hydrocarbons which are susceptible to oxidation, such as rubbers, plastics, foods, and oils to inhibit or slow oxidative processes, while being itself oxidized. Antioxidants work in two different ways. In primary antioxidants (also called free-radical scavengers), antioxidative activity is implemented by the donation of an electron or hydrogen atom to a radical derivative. These antioxidants are usually hindered amines (p-Phenylene diamine, trimethyl dihydroquinolines, alkylated diphenyl amines) or substituted phenolic compounds with one or more bulky functional groups such as a tertiary butyl at 2,6 position commonly. Butylated hydroxytoluene (BHT) is a common example of hindered phenolic antioxidant. The reaction rate, or carbocation stability, in SN1 mechanism is 3° > 2° > 1° > CH3 (no SN1) so, tertiary alkyl moiety exists in lots of phenolic antioxidant compounds. Primary antioxidants are free radical scavengers which combine with peroxy radicals and break autocatalytic cycle. In secondary antioxidants ( also called peroxide decomposers), activity is implemented by the removal of an oxidative catalyst and the consequent prevention of the initiation of oxidation. Examples of peroxide decomposer type of antioxidant are trivalent phosphorous and divalent sulfurcontaining compound such as sulfides, thiodipropionates and organophosphites. Synergistic effect is expected when primary antioxidants are used together with secondary antioxidants as primary antioxidants are not very effective against the degradation by UV oxidation. Sometimes, chelating agents are added to scavenge metal impurities which can initiate decomposition.
SALES SPECIFICATION
APPEARANCE
99.0% min
MELTING POINT
285 C min
MOISTURE
0.1% max
ASH
0.5% max
HEAT LOSS
0.3% max
GENERAL DESCRIPTION OF THIOL
The first chemical contrast of thiols and sulfides with alcohols and ethers is acidity which is important in organic reactions. Thiols are stronger acids than relevant alcohols and phenols.Thiolate conjugate bases are easily formed, and are excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. The nucleophilicity of sulfur is much greater than that of oxygen, resulting in a number of useful electrophilic substitution reaction that are rare by oxygen. For example, sulfides form (with alkyl halides) ternary sulfonium salts, in the same alkylattion of tert-amines quaternary ammonium salts, whereas ternary oxonium salts are prepared only under extream conditions. Without exception, sulfoxides, sulfinate salts and sulfite anion also alkylate on sulfur, despite of the partial negative formal charge on oxygen and partial positive charge on sulfur. The second character is the oxidation states of sulfur. Oxygen has only two oxidation states, whereas sulfur covers from –2 to +6 as follows:
- -2: Hydrogen Sulfide (H2S), sulfides, sulfonium ions
- -1: disulfides
- 0: S elemental, sulfoxides, sulfenic acids
- +2: sulfones, sulfinic acids
- +4: sulfonic acids, sulfite esters
- +6: sulfate esters
One more sulfur compound's contrast with oxygen analog is in oxidation chemistry. Oxidation of sulfur compounds changes the oxidation state of sulfur rather than carbon, whereas, oxidation of alcohols to aldehydes and ketones changes the oxidation state of carbon not oxygen. Thiols is oxidized to S-S single bond (disufide) which is stronger than O–O bond in peroxide. Disufide forms sulfenyl chlorides (with chlorine in mild condition) or sulfonic acids under harder condition. Oxidation of sulfides with hydrogen peroxide (or peracids) yields sulfoxides and then to sulfones. A certain sulfoxide compound such as dimethyl sulfoxide can be used as an effective oxygen source in the oxidation reaction of primary and secondary alcohols to aldehydes and ketones. DMSO easily is reduced to dimethyl sulfide and water is taken up by the electrophile. oxidation procedure is very mild and tolerates a variety of other functional groups, including those having oxidizable nitrogen and sulfur atoms.