PRODUCT IDENTIFICATION
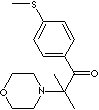
279.41
2-Methyl-1-(4-Methylthio)phenyl-2-Morpholinyl-1-Propanon;
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
APPLICATIONS
- Peroxides including hydroperoxides (tertiary-butyl hydroperoxide, benzoylperoxide)
- Azocompound thermal initiators (azoisobutyronitrile)
- Redoxinitiators (mixture of iron(III) acetylacetonate): free radicals are formed by one-electron transferreactions. Useful in low temperature and emulsion polymerization
- Photoinitiators (benzoin, benzil dimethylketal)
The main advantage of polymerization started by photoinitiators is temperature-independence and easy control. It can be conducted at very low temperatures and can be stopped simply by removing the light source. Photoinitiators are compounds that break down into free radicals upon exposure to ultraviolet radiation. Photoinitiators undergo a unimolecular bond cleavage upon irradiation to yield free radicals (benzoin esters; benzil ketals; alpha-dialkoxy acetophenones; alpha-hydroxy-alkylphenones; alpha-amino alkyl- phosphine; acylphosphine oxides). Another type of photoinitiators undergo a bimolecular reaction where the excited state of the photoinitiator interacts with a second molecule (a coinitiator) to generate free radicals(benzo phenones,amines; thioxanthones,amines; titanocenes). Photoinitiators are widely applied in UV curing inks, wood coatings, paper coatings, optical fiber, PCB, screen printing , paper varnish and other surface coatings.
APPEARANCE
ASSAY
98.0% min
VOLATILES
0.3% max
CLARITY OF SOLUTION
Clear ( 10g in 100ml acetone)
TRANSMITTANCE
70% min (425nm) , 85% min (500nm)
- Rubber chemicals for vulcanization and stabilization
- Corrosion inhibitors in boiler water treatment system and in aqueous hydraulic liquids to protect metals against corrosion and tarnish by acid fumes.
- Optical brighteners which are stable to chlorine bleaches for the use in detergent formulations
- Fatty acid salts for the formulation of water-resistant emulsifier or plasticizer in toiletry and cosmetic products.
- Sulfonamide bactericides or disinfectants
- Quaternary morpholinium salts for hair conditioners and deodorant products
- Colourants for hair dyes and blueprints
- Pharmaceuticals (analgesics, local anaesthetics, antibiotics, antimycotics and for anti-plaques)