PRODUCT IDENTIFICATION
91-59-8
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
yellow crystalline
1.05 - 1.10
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
yellow powder
99.0% min
0.5% max
|
|
|
| 2-NAPHTHYLAMINE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
91-59-8 |
|
| EINECS NO. | 202-080-4 | |
| FORMULA | C10H7NH2 | |
| MOL WT. | 143.19 | |
| H.S. CODE | 2921.45 | |
| TOXICITY | Oral rat LD50: 779 mg/kg | |
| SYNONYMS | 2-Aminonaphthalene; beta-Naftalamin; | |
| beta-Naphthylamine; NA; 2-Naphthalenamine; BNA; Fast Scarlet Base B; 6-naphthylamine; C.I. 37270; 1-Naphthalenamine; | ||
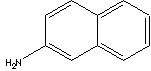
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
yellow crystalline |
|
| MELTING POINT | 111 - 113 C | |
| BOILING POINT | 300 C | |
| SPECIFIC GRAVITY |
1.05 - 1.10 |
|
| SOLUBILITY IN WATER | Soluble (soluble in alcohol and ether) | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions ( light sensitive) | |
|
APPLICATIONS |
||
| Naphthylamine is an aromatic amine which can be obtained from nitronaphthalene (with iron and hydrochloric acid) or naphthol with sodium acetate, ammonium chloride. There are position isomers; 1-naphthylamine and 2-naphthylamine. 2-Naphthylamine was used as an Intermediate for wide application target molecules including dyestuffs, agrochemicals, and antioxidants. But its use is restricted or prohibited due to cancer suspect. Naphthylamine is the naphthalene homologues of aniline. Naphthylamine is the starting material in the dye manufacturing and rubber industry. Numerous sulfo- and nitro-group substituted naphthylamine on the ring system offer important roles to each characteristic colours directly and in preparing next target colorants. Phenyl group substituted naphthylamines are naphthalene homologues of phenylenediamines used as parent compounds in vulcanization accelerators and in antioxidants for rubber industry. Naphthylamine can be oxidized by chromic acid into naphthoquinone which is the fundamental ring structure related with vitamin K. Tetrahydronaphthylamine can be oxidized by potassium permanganate into adipic acid. Naphthyl structure is found as a ligand in transition-metal catalyts particularly in the form of bi-naphthyl which is composed of two naphthyl rings connected at one carbon site on each ring. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow powder |
|
| CONTENT |
99.0% min |
|
| ISOMER IMPURITY |
0.5% max |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
|
||
|
|
|