PRODUCT IDENTIFICATION
CLASSIFICATION
AROMATIC AMINES / NITRO COMPOUNDS / DYES /
PHYSICAL AND CHEMICAL PROPERTIES
yellow to brown crystal
AUTOIGNITION
REFRACTIVE INDEX
168 C
GENERAL DESCRIPTION & APPLICATIONS
- Michael addition
- Reduction
- Henry Reaction (Nitro-aldol reaction)
- Nef reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion reaction
- Alkylation, Acylation, and Halogenation
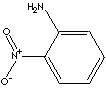
One of the most important aromatic amines is aniline; pale brown liquid boiling at 184 C, melting at -6 C. Aniline is obtained commercially from chlorobenzene by heating with ammonia in the presence of copper catalyst or from a product of coal tar (nitrobenzene) through the reduction reaction. Aniline is the starting material in the dye manufacturing industry and as in the manufacture of others. Aniline is converted into sulfanilic acid which is the parent compound of the sulfa drugs. Aniline is also important in the manufacture of rubber-processing chemicals, antioxidants and varnishes. 2-Nitroaniline is used in the synthesis of dyes, agrochemicals, pharmaceuticals, rubber and plastic additives, photographic antifogging agents and coccidiostats.
APPEARANCE
yellow to brown crystal
85.0% min