PRODUCT IDENTIFICATION
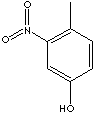
2908.90
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
Brown Solid
REFRACTIVE INDEX
APPLICATIONS
- Michael addition
- Reduction
- Henry Reaction (Nitro-aldol reaction)
- Nef reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion reaction
- Alkylation, Acylation, and Halogenation
Mononitrophenols are used as intermediates for the synthesis of a number of organophosphorus pesticides, azo dyes, and some medical products as well as their corresponding amonophenols by reduction. some end products derived from mononitrophenols include dyes and pigments, acetaminophen (from p-aminophenol), carbofuran, phosalon (insecticides form o-nitrophenol), parathion,parathion-methyl, fluorodifen (insecticides form p-nitrophenol), nitrofen, bifenox (herbicides form p-nitrophenol), fungicides, and rubber chemicals. Nitrophenol is used as a pH range indicator (colorless at 5 and yellow at 7). Nitrophenol derivatives are used as intermediates for the synthesis of a number of target products.
APPEARANCE
Brown crystalline powder
98.0% min
MELTING POINT