PRODUCT IDENTIFICATION
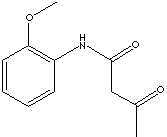
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
85 C
REFRACTIVE INDEX
APPLICATIONS
Acetoacetates have a reactive hydrogen atom on the carbon alpha to both carbonyl groups. It undergoes Knoevenagel condensation reaction as a reactant to forms a large class of target products including amino acids, drugs, colorants, lacquers, perfumes, and plastics. Alone, it is used as a flavoring agent and a solvent. Knoevenagel condensation is a nucleophilic addition of a reactive hydrogen atom at 1,3-diketone compounds to a carbonyl group, followed by an dehydration reaction. 1,3-Diketone compounds (beta-ketones) include malonic acid, diethyl malonate, Meldrum's acid, and acetoacetic acid derivatives.
AAOT is used in manufacturing agricultural chemicals, coating materials, dyes and pigments, pharmaceuticals, co-promoters for polymers. Anilide is a amide in which one or more hydrogens are replaced by phenyl; having the C6H5NH2-group.
Acetoacetaniside is an intermediate for organic yellow pigments, diazo pigment complex with bisdiazonium salt compounds. Anisic acid, p-methoxybenzoic acid, is a part of cresol class antiseptic compounds. It is also used as an insect repellent and ovicide. Anisole, anisic acid, and their deivatives are also widely used in chemical reaction as intermediates to obtain target materials such as dyes, pharmaceuticals, perfumes, photoinitiators and agrochemcials.
APPEARANCE
0.2% max