PRODUCT IDENTIFICATION
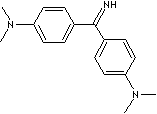
C(c1ccc(N(C)C)cc1)(c1ccc(N(C)C)cc1)=N
CLASSIFICATION
EXTRA NOTES
Overall Carcinogenic Evaluation: Group 2B
EPA Pesticide Chemical Code 039501
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Solvent dye
Google Scholar Search - Auramine
Drug Information Portal (U.S. National Library of Medicine) - Auramine base
PubChem Compound Summary - Auramine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Auramine base
http://www.ebi.ac.uk/ - Auramine base
http://www.ncbi.nlm.nih.gov/ - Auramine base
http://toxnet.nlm.nih.gov/
Hazardous
Substances Data Bank - Auramine
base
http://monographs.iarc.fr/
Auramine is manufactured industrially from N,N-dimethylaniline and formaldehyde,
which react to form Michler��s base (tetramethyldiaminodiphenylmethane). This base is subsequently converted to auramine by heating with sulfur and ammonium chloride in the presence of ammonia. It was reported that a 98% pure auramine contains salts, water and Michler's ketone, an hydrolysis product (Kirsch et al.,1978). Production of auramine took place first in Europe (Switzerland, Germany, the United Kingdom, and France), and later also in the United States of America (USA). Production in these countries has generally been discontinued. Auramine manufacturing is currently mainly located in India and the People��s Republic of China.Auramine colourants are used for dyeing of leather, jute, tanned cotton, and paints, and as
dye components in inking ribbons, ballpoint pastes, oils and waxes, and carbon paper. The most important applications are in paper dyeing and flexographic printing (IARC, 2010). More detailed information on the use of auramine dyes and auramine compounds is provided in the recent Monograph
APPEARANCE
STRENGTH
100.0%
MOISTURE
3.5% max
1.5% max
9 (Packing group: III)
3077
HAZARD OVERVIEW
May be harmful if swallowed. Causes serious eye irritation. Suspected of causing cancer. Toxic to aquatic life.
GHS
PICTOGRAMS



HAZARD STATEMENTS
H302-H319-H351-H411
P STATEMENTS
P273-P281-P305 + P351 + P338
![]()
![]()
RISK PHRASES
22-36-40-51/53
SAFETY PHRASES
36/37-61