PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
APPLICATIONS
Sulfoxide (R2SO) is any of various organic sulfur compounds having the group -SO (sulfinyl group) whereas sulfone (RSOOR) with the group -SO2 (sulfonyl group). They are derived from oxidation of sulfides. They are widely used as solvent of both extraction and reaction as well as intermediates for the synthesis of textile chemicals and pharmaceuticals and agrochemicals.
Organosulfone compounds are widely used in refineries, steamcrackers, aromatic extraction and petrochemical manufacturing as they acts as;
- Hydrotreating catalysts
- Initial catalyst improvers
- Sulfur sources
- Catalyst presulfiding
- Natural gas sulfur recovery
Organosulfone compounds are also used in;
- Organic synthesis as organosulfur sources into target organic molecules for the manufacture of pharmaceuticals, adhesives biocides and agricultural products.
- Polymer production
- Lubricant and fuel additives for extreme pressure functionality
- Extraction and reaction solvent
- Metal treatment
- Fungicide
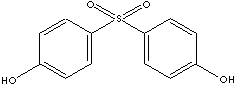
Bisphenol S, hydroxy substituted organosulfone compound, is used in preparing for:
- Developer for heat sensitive paper; sulfone polymers; fire retardants
- Couplers for photography; tanning agents
- Electroplating chemicals
- Intermediate of colorants, pharmaceuticals (antileprotic drugs) and other compounds
- Modifiers for leather, fiber and polymers, epoxy curing agents.
APPEARANCE
99.5% min
MELTING POINT
239 - 248 C
MOISTURE
0.1% max