PRODUCT IDENTIFICATION
EINECS NO.
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
0.852
NFPA RATINGS
AUTOIGNITION
207 C
REFRACTIVE INDEX
1.437
13 C
APPLICATIONS
APPEARANCE
99.0% (or 92.0%)
ACIDITY
0.2% max
WATER
0.5% max
|
|
|
| CROTONALDEHYDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 4170-30-3, 123-73-9 |
|
|
EINECS NO. |
204-647-1 | |
| FORMULA | CH3CH=CHCHO | |
| MOL WT. | 70.09 | |
| H.S. CODE | 2912.19 | |
| TOXICITY | Oral rat LD50: 80 mg/kg | |
| SYNONYMS | propylene aldehyde; Diproanoate; Crotylaldehyde; | |
| beta-methylacrolein; Crotonic aldehyde; But-2-enal; 2-Butenal; crotonal; topanel; methyl acrolein; Butenal; 2-Butenal (cis+-trans); 1-Formylpropene; Krotonaldehyd; Methylpropenal; | ||
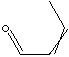
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear to yellow liquid | |
| MELTING POINT | -76 C | |
| BOILING POINT | 101 - 102 C | |
| SPECIFIC GRAVITY |
0.852 |
|
| SOLUBILITY IN WATER | soluble | |
| pH | ||
| VAPOR DENSITY | 2.41 | |
|
NFPA RATINGS |
Health: 4 ; Flammability: 3 ; Reactivity: 3 | |
|
AUTOIGNITION |
207 C |
|
|
REFRACTIVE INDEX |
1.437 |
|
| FLASH POINT |
13 C |
|
| STABILITY | Air and light sensitive. Forms explosive mixtures with air | |
|
APPLICATIONS |
||
| Aldehydes are organic compounds containing -CHO radical, in which a carbon atom forms a solid bond with an oxygen atom and is also bonded to a hydrogen atom and another group denoted by R, which can be a second hydrogen atom, an alkyl group, or an aryl group. The most important and the simplest examples are methanal (formaldehyde), HCOH, and ethanal (acetaldehyde), CH3CHO.( In systematic chemical nomenclature, aldehyde names end with the suffix -al). Formaldehyde is used to make synthetic resins by reaction with phenols, urea, and melamine, as a chemical intermediate, as an embalming fluid, and as a disinfectant. Acetaldehyde is used chiefly to manufacture acetic acid. They are unpleasant-smelling liquids widely used in the chemical industry, whereas aromatic aldehydes frequently have pleasant smells and are used widely as flavourings and perfumes. An example is benzaldehyde (benzenecarbaldehyde, C6H5CHO), a derivative of benzene with an aldehyde group attached to the ring. It is a colourless oil smelling of almonds offer applications to both in perfumes and flavourings. Aldehydes are formed by oxidation of primary alcohols; further oxidation yields carboxylic acids. Aldehydes have certain characteristic addition and condensation reactions. Aldehydes can be reduced to primary alcohols (ketones to secondary alcohols). Aldehydes form cyanohydrins with hydrogen cyanide, acetals with alcohol, yellow-orange solid derivatives with DNP ( 2,4-dinitrophenylhydrazones) and undergo condensation reactions to yield oximes (compounds containing the group C:NOH), hydrazones (organic compounds containing the group =C:NNH2), and semicarbazones (organic compounds containing the unsaturated group =C:N.NH.CO.NH2). Aldehydes readily polymerize. Crotonaldehyde, C-4 aldehyde containing a solid bond, is a very reactive liquid. The mixtures with air is highly flammable. It is a clear to pale yellow highyl flammable liquid with with a pungent odour and lachrymation effect. It turns pale yellow on exposure to light and air. This substance is a strong reducing agent and reacts violently with oxidants and many other substances. It can polymerize and can cause fire and explosion hazard. The vapour is severely irritating to the skin, the respiratory tract and corrosive to eyes. Inhalation of high concentrations may cause lung oedema and death eventually. Crotonaldehyde is commercially prepared by aldol condensation from acetaldehyde. The solubility in water is poor. But it is very soluble in organic solvents including alcohols and ether. Crotonaldehyde form an azeotrope boiling point at 84 C with 25% water. Crotonaldehyde is used as a solvent for vegetable and mineral oils, fats, and resins as well as for certain sulfur compounds. Its application finds in resins as a co-monomer or as a flow-promoting agent. Crotonaldehyde is used as a denaturing agent with the addition to alcohol to render it unfit for drinking, or the change in the physical properties of a substance. It is used to detect gas leaks in pipes. Due to bi-functional group of aldehyde and solid bond within C-4 chain, crotonaldehyde is effectively used as a starting product for the numerous organic reactions and synthesis. The main use of crotonaldehyde is to prepare sorbic acid and crotonic acid by oxidation. It is used in the synthesis of n-butyl alcohol, butyraldehyde and its analogues by reduction. Crotonaldehyde forms alkoxy butyraldehyde and alkoxy butanol in subsequent reduction and hydrogenation. It is a raw material to produce quinaldines, thiophenes and pyridines for the manufacturing finish products including oil soluble dyes, food colorants, pesticides, pharmaceuticals and pH indicators. Crotonaldehyde is a raw material for a slow-release fertilizers like crotonylidene ureas. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellow liquid | |
| ASSAY |
99.0% (or 92.0%) |
|
|
ACIDITY |
0.2% max |
|
|
WATER |
0.5% max |
|
| TRANSPORTATION | ||
| PACKING | 185kgs in Drum | |
| HAZARD CLASS | 3(9.2) , (Packing group: I) | |
| UN NO. | 1143 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T F, Risk Phrases: 11/23/36/37/38, Safety Phrases: 29/33/45 | ||
|
|
|