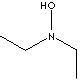
DIETHYLHYDROXYLAMINE
PRODUCT IDENTIFICATION
3710-84-7
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
1.420
46 C
APPLICATIONS
Hydroxylamine is a white crystalline compound containing nitrogen with the formula of NH2OH and is therefore an ammonia (NH3) like compound. In the nature, hydroxylamine is an biological intermediate in the nitrification (biological oxidation of ammonia with oxygen into nitrite ) and in the anammox (biological oxidation of nitrite and ammonium into dinitrogen gas) which are important in the nitrogen cycle in soil and in wastewater treatment. Hydroxylamine is obtained commercially by acid hydrolysis of nitroparaffins or by the modified reduction of nitric acid. It is used as a powerful reducing agent in photography and in organic synthesis. It converts aldehydes (and ketones) to oximes (caprolactam), and acid chlorides to hydroxamic acids. It is used as a catalyst or inhibitor in polymerization processes. It is used in enzyme reactivation. It is used as an antioxidant in fatty acids and soaps. The nitrate form of hydroxylammonium is used as a rocket fuel which is burned with an oxidizer to produce thrust. Hydroxylamine tends to be explosive when heated. Its derivatives in the form of salts are more stable to be used and handled. Hydroxylamine Hydrochloride is used as an auxiliary in photographic industry to prevent discolouration. It is a polymerization inhibitor or free radical scavenger against solid bond monomers such as olefin, styrene, butadiene, isoprene and divinylbenzene. It is also used in rubber synthesis processes as a non-discoloring short stoppers.
Hydroxylammonium sulfate similar applications to hydrochloride salt in the field of :
- Organic synthesis: preparation of oximes, hydroxamic acids from carboxylic acids, N- and O- substituted hydroxyamines, and addition reactions of carbon-carbon solid bond.
- Surface treatments: preparation of anti-skinning agents, corrosion inhibitors, meta; cleaner additive
- Starting material for pharmaceuticals and agrochemicals manufacturing
- Rubber and plastic industry: antioxidant, vulcanization accelerator, radical scavenger.
- Textile industry: fixative for textile dyes, auxiliary in some dyeing processes. bleaching
- Metallurgy: Metal extraction and flotation aid
- Aantioxidant in fatty acids and soaps
- Photographic auxiliary as a stabilizer of colour and emulsion additive for colour films.
Chemical oxygen scavengers are used to scavenge remaining oxygen water to prevent the corrosion in the closed water system. Oxygen scavengers include several organic and inorganic types such as hydrazine, hydroquinone, diethylhydroxyethanol, oximes, and sulfites. Sulfite reacts with remaining oxygen and forms sulfate. Hydrazine reacts with remaining oxygen to generate water and nitrogen. Diethylhydroxylamine, nitrogen-containing compound, has the property of oxygen scavenging and used in water treatment chemical formulation to prevent corrosion. It is used as an auxiliary in photographic industry to prevent discolouration. It is a vapour phase polymerization inhibitor against solid bond monomers such as olefin, styrene, butadiene, isoprene and divinylbenzene. It is used as a stabilizer for phenolics. It is used as an intermediate for pharmaceuticals and water treatment chemicals. It is also used as a raw material of silicon sealant and coating materials.
APPEARANCE
98.0% min
IMPURITY
85% AQ. SOLUTION
PURITY
85.0% min
WATER
15.0% max
DIETHYLAMINE