|
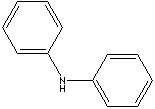
DIPHENYLAMINE | ||
|
PRODUCT IDENTIFICATION |
||
|
CAS NO. |
122-39-4 |
|
| EINECS NO. | 204-539-4 | |
| FORMULA | (C6H5)2NH | |
| MOL WT. | 169.23 | |
| H.S. CODE | 2921.44 | |
| TOXICITY | Oral rat LD50: 2000 mg/kg | |
| SYNONYMS | N-Phenylbenzenamine; N-Phenyl Aniline; DPA; | |
| Anilinobenzene; (phenylamino)benzene; N,N-diphenylamine; big dipper; C.I. 10355; Phenylbenzenamine; Diphenylamine ; | ||
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White to yellow crystals | |
| MELTING POINT | 52 C | |
| BOILING POINT | 302 C | |
| SPECIFIC GRAVITY |
1.16 | |
| SOLUBILITY IN WATER | Slightly | |
| AUTOIGNITION |
634 C | |
| pH | ||
| VAPOR DENSITY | 5.82 | |
| NFPA RATINGS | Health: 3 Flammability: 1 Reactivity: 0 | |
| FLASH POINT |
152 | |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Amine is a group
of basic organic compounds derived from ammonia (NH3) by replacement
of one (primary amines), two (secondary amines), or three (tertiary amines)
hydrogen atoms by alkyl, aryl groups or organic radicals. Most
low mole weight aliphatic amines are liquids with characteristic ammonia-like
odor. But methylamines are gases and long chain alkylamines (from C12) or higher
mole weight amines are
solid. The water solubility is decreasing if molecular weight increase. They
are freely soluble in common organic solvents such as methanol, acetone,
toluene, and ether except aliphatic hydrocarbons. Amines, like ammonia,
are weak bases because the unshared electron pair of the nitrogen atom can form
a coordinate bond with a proton. Amines react with acids to give salts and with
acid anhydrides (or ester ) to form amides. They react with halogenoalkanes to
form longer chains.
Many amines are not only bases but also nucleophiles that form a variety of electrophile compounds. They are important intermediates for chemical syntheses due to the basic functionality of the nitrogen atom and electrophilic substitution at nitrogen. Some examples of compounds obtained by reaction of amines are:
Low molecular amine names are formed by adding '-amine' as a suffix to the name of the parent compound. In substitutive nomenclature, the prefix 'amino-' is placed before the name of the parent compound to denote the functional group in high molecular amines. Synthetic amines are made mostly by reaction of alcohols with ammonia, catalyzed by metals( nickel or copper) or metal oxide at high temperature. Many methods have been devised for the synthesis of the amines; reacting ammonia with an alkyl halide and neutralizing the resulting alkyl ammonium salt with an alkali, e.g., sodium hydroxide. This procedure yields a mixture of primary, secondary, and tertiary amines that is easily separated into its three components by fractional distillation; boiling methyl isocyanate with caustic potash, heating the alkyl iodides with ammonia; reduction of nitriles with alcohol and sodium; heating the esters of nitric acid with alcoholic ammonia; reducing on nitro-paraffms; action of zinc and hydrochloric acid on aldehyde ammonias; reduction of the phenylhydrazones and oximes of aldehydes and ketones with sodium amalgam in the presence of alcohol and sodium acetate; action of dilute hydrochloric acid on the isonitriles; heating the mustard oils with a mineral acid, by the hydrolysis of the alkyl phthalimides. Primary amines contain the functional group -NH2 (called amino group) and are converted into secondary and tertiary amines if heated with alkyl or aryl iodides. Primary amines form various oxidation products violently with concentrated nitric acid. If the amines are acetylated, they form nitro derivatives with concentrated nitric acid. Primary amines form diazonium salts with nitrous acid in cold solution in the presence of excess of mineral acid. Or a diazoamine is obtained in absence of excess of acid. Other reactions are condensation products with aldehydes; forming anilides; forming alkyl thioureas; yielding isonitriles with alcoholic potash and chloroform. Tertiary amines combine with one molecular proportion of an alkyl iodide to form quaternary ammonium salts in which a central nitrogen atom is joined to four organic radicals and one acid radical. Quaternary ammonium salts are used as corrosion inhibitor, emulsifying and antiseptic agents. Aliphatic amines which have the lowest carbon content are water-soluble gases or liquids of low boiling point also readily soluble in water in case of the next low carbon content. But aliphatic amines which have the high carbon content are odourless solids of high boiling point and are insoluble in water. They are all bases and easily form salts with the mineral acids and solid salts with the halogenoalkanes. Amine Salts are crystalline substances that are readily soluble in water. Many insoluble alkaloids (e.g. quinine and atropine) are used medicinally in the form of soluble salts. If alkali (sodium hydroxide) is added to solutions of such salts the free amine is liberated. Short chain alkyl amines are used as raw materials of solvent, alkyl alkanolamines, and ingredients of rocket fuels. They are used to make other organic chemicals including rubber vulacanization accelerators, pesticides, quaternary ammonium compounds, photographic chemicals, corrosion inhibitors, explosives, dyes and pharmaceuticals. They are used in rayon and nylon industry to improve the tensile strength. Allylamines are used as intermediates for ion exchange resins, pharmaceuticals, water soluble polymers, herbicide softeners, rubber chemicals, polymerization initiators and cross-linking agents. Amines are used as reducing agents for the recovery of precious metals. They are versatile intermediates. They have active applications in organic synthesis for polymerization catalyst, chain extender in urethane coatings, agrochemicals, pharmaceuticals, photographic, heat stabilizers, polymerization catalysts, flame-retardants, blowing agents for plastics, explosives, and colorants. Long chain alkyl amines are used for the synthesis of organic chemicals and surfactants used as a corrosion inhibitor, detergent, ore floating agent, fabric softener, anti-static agent, germicide, insecticide, emulsifier, dispersant, anti-caking agent, lubricant and water treatment agent. Alkyl tertiary Amines are used as fuel additives and preservatives. They have similar applications with long chain alkyl amines. Hexamethylenediamine used in the manufacture of nylon-6,6 is prepared by catalytic addition of hydrogen to nitriles. Aromatic amines also exist, such as phenylamine and benzylamine, which are important for the production of diazonium salts. They dissociate in water (some very weakly). Aromatic amines are much weaker bases than the aliphatics. The term benzyl describes the radical, ion or functional group C6H5CH2-, derived by removing hydrogen atom from methyl group in toluene, while phenyl is the term for the monovalent radical C6H5-, derived by removal of hydrogen from benzene. The common name of phenylamine is aniline. Benzylamine is called aminotoluene or benzenemethanamine in methane nomenclature system. Benzylamine functions in the way as primary aliphatic amines. Benzylamine is a clear liquid boiling at 185 C. It functions in the same way as primary aliphatic amines. Benzylamine and its derivatives are used as chemical intermediate for the manufacture of dyestuffs, pigments, optical brighteners, textile auxiliaries, agrochemicals, amino acids and other organic compounds. One of the most important aromatic amines is aniline, a primary aromatic amine replacing one hydrogen atom of a benzene molecule with an amino group. It is a pale brown liquid at room temperature; boiling at 184 C, melting at -6 C; slightly soluble in water and freely soluble in ether and alcohol. It causes serious industrial poisoning. The substance may have effects on the blood, resulting in formation of methaemoglobin. Repeated or prolonged exposures may be carcinogenic. Commercial aniline is obtained from nitrobenzene which is prepared from benzene with nitric acid by electrophilic substitution reaction or from chlorobenzene by heating with ammonia in the presence of copper catalyst. It is also obtained as a by-product of coal tar. In commerce the term of aniline oil blue refers to the pure one while aniline oil red indicates a mixture of aniline and toluidines with equimolecular weights. Considerable quantity of aniline is converted into 4,4��-methylenedianiline (MDA) by the condensation reaction of formaldehyde with aniline in the presence of hydrochloric acid. MDA is is used as an epoxy curing agent, a corrosion inhibitor and molded plastics, and as an intermediate to prepare organic compounds used for polyurethane, spandex fibers, azo dyes, isocyanates and poly(amide-imide) resins. Other important aromatic amine compound as the starting material to produce polyurethane foam production is toluenediamine (TDA). TDA is the mixture of 2,4-diaminotoluene and 2,6-diaminotoluene, usually in a ratio of 80:20. Most of TDA is used in the manufacture of toluene diisocyanate (TDI), which is the predominant diisocyanate in the flexible foams and elastomers industries. TDI reacts with an alcohol to form urethane linkages. Other applications of TDA include to produce dyes, polyamides, antioxidants, hydraulic fluids, and fungicide stabilizers. Aniline is a starting moiety to prepare plant protecting agents. Examples include fenuron (CAS RN: 101-42-8), propham (CAS RN: 122-42-9), siduron (CAS RN: 1982-49-6), carboxin (CAS RN: 5234-68-4), fenfuram (CAS RN: 24691-80-3) and propachlor (CAS RN: 1918-16-7). Aniline is processed to produce a series of compounds being used in the rubber industry, e.g. diphenylguanidines, phenylenediamines mercaptobenzothiazoles, aniline ketones and etc. There are three isomers of phenylenediamine: ortho-, meta-, and para-phenylenediamine. They are low toxic diamines used as components of plastic composites and engineering polymers. They are used to produce aramid fibers, dyes including hair dyes, rubber chemicals (vulcanization accelerators and antioxidants), and pigments. Aniline is the starting material in the dye manufacturing industry. It forms aniline colors when combined with other substances, particularly chlorine or chlorates. Aromatic amines are weaker bases reacting with strong acids to form amides. Anilide is an amide derived from aniline by substitution of an acyl group for the hydrogen of NH2. Acetanilide is from acetic acid and aniline. Acetanilide is an odourless, white flake solid or crystalline powder (pure form); soluble in hot water alcohol, ether, chloroform, acetone, glycerol, and benzene;; melting point 114 C and boiling point 304 C; can undergo self-ignite at 545 C, but is otherwise stable under most conditions. Acetanilide which can be obtained by acetylation of aniline undergoes nitration at low temperature and yields highly the para-nitro products. Acetyl group can then be removed by acid-catalyzed hydrolysis to yield para-nitroaniline. Although the activating affection of the amino group can be reduced, the acetyl derivative remains an ortho/para-orientation and activating substituent. Aniline is converted into sulfanilic acid which is the parent compound of the sulfa drugs. Aniline is also important in the manufacture of rubber-processing chemicals, explosives, plastics, antioxidants and varnishes. Amines take part in many kinds of chemical reactions and offer many industrial applications. The applications and properties of diphenylamine are similar to those of aniline. Diphenylamine is used as a parent compound for vulcanization accelerators and for antioxidants (and antiozonant) for rubber industry. These derivatives are added to rubber products to retard degradation by oxidants and ozone. They are intermediates for the production of dyes, pH indicators, polymers and fibers, and curing agents. Diphenylamine is used as a test for oxidizing agents and in detecting DNA in labs. Diphenylamine is used to absorb decomposed catalysts in solid fuel rocket propellant. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White to yellow crystals | |
| ASSAY |
99.2% | |
| CRYSTALLIZING POINT |
51.7 C min | |
| MOISTURE |
0.2% max | |
| TOLUENE INSOLUBLES |
0.02% max | |
| ACIDITY (AS HCl) |
0.005% max | |
| ALKALINITY (AS NaOH) |
0.005% max | |
| ANILINE |
0.1% max | |
|
4-AMINODIPHENYL |
0.01% max | |
|
QUINOLINE |
0.2% max | |
|
ACRIDINE |
0.3% max | |
| INDOLE | 0.5% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in Bag | |
| HAZARD CLASS | 9 | |
| UN NO. | 3077 | |
| OTHER INFORMATION |
||