PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white solid
REFRACTIVE INDEX
151 C
GENERAL DESCRIPTION AND APPLICATIONS
- Acenaphthene: Intermediate for naphthalic acids, naphthalic anhydride (intermediate for pigments) and for acenaphthylene (intermediate for resins); Intermediate for dyes, soaps, pigments, pharmaceuticals, insecticide, fungicide, herbicide and plant growth hormones. It is used to manufacture plastics and as an agent for inducing polyploidy.
- Acridine: Dye and pharmaceutical manufacturing
- Anthracene: Its oxidation yields anthraquinone, the parent substance of a large class of dyes and pigments; .diluent for wood preservatives; scintillant (for detection of high-energy radiation)
- Fluoranthene: manufacturing fluorescent and vat dyes, pharmaceuticals and agrochemicals.
- Fluorene: basic subsance for production of dyes, pigments, pesticides, thermosetting plastic and pharmaceuticals; manufacturing fluorenone (mild oxidizing agent)
- Naphthalene: In the production of phthalic anhydride, carbaryl insecticide, beta-naphthol, tanning agents, moth repellent, and surfactants - naphthalene: main use: production of phthalic anhydride (intermediate for polyvinyl chloride plasticizers); also, production of azo dyes, surfactants and dispersants, tanning agents, carbaryl (insecticide), alkylnaphthalene solvents (for carbonless copy paper), and use without processing as a fumigant (moth repellent)
- Phenanthrene: manufacturing phenanthrenequinone (intermediate for pesticides); manufacturing diphenic acid (intermediate for resins)
- Pyrene: manufacturing perinon pigments
- Quinoline: solvent for resins & terpines; decarboxylation agent; parent compound to make drugs, fungicides, biocides, alkaloids, dyes, rubber chemicals and flavoring agents
Precise PAHs, specific refined products are used also in the field of electronics, functional plastics and liquid crystals. Pharmaceutical and agricultural PAHs obtained coal tar are such materials as indole, indolizine, indene, quinoline, quinalidine, isoquinoline and their derivatives. High boiling-point special solvent are such materials as tetoralin, decaline, methyl-naphthalenes. Coumarins and dihydrocoumarins which can be obtained coal tar are PAHs used in perfumery. Thermosensitive paper sensitizer PAHs are such materials as p-benzylbiphenyl and ethylbiphenyl.
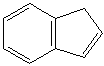
Fluorene is a member of polycyclic aromatic hydrocarbon (PAH). Two benzene rings are fused to cyclopentane ring. It emits violet fluorescent color. It is not synthesized commercially but is obtained from middle oil fraction of coal tar. It is insoluble in water; soluble in ether and acetone; melting point 116-117 C. It plays important part in metallocene catalysts as a ligand. It is used in the formation of polyradicals for resins. It is used in manufacturing antimalaria drugs and other pharmaceuticals. Fluorene family compounds are base materials for dyes and optical brightening agents. They have useful functions such as light and temperature sensitivity, heat resistance, conductivity, emittability, corrosion resistance and detection of amino groups. They are used in the applications of thermo and light sensitizer, liquid crystal chemistry, luminescence chemistry, spectrophotometric analysis, molecular chemistry, organometallic-complexes and biochemorphology industry.APPEARANCE
97.0% min
MELTING POINT
115 ± 1 C
LOSS ON DRYING
0.5% max
99.0% (50nm, 10% Toluene Sol.)
1224
EXAMPLES OF PARENT PAH COMPOUNDS
|
|
|
|
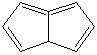
PENTALENE |
INDENE (CAS RN: 95-13-6) |
|
|
|
|
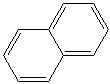
NAPHTHALENE (CAS RN: 91-20-3) |
AZULENE (CAS RN: 275-51-4) |
|
|
|
|
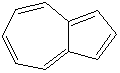
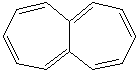
HEPTALENE |
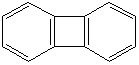
BIPHENYLENE (CAS RN: 259-79-0) |
|
|
|
|
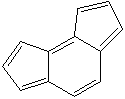
as-INDACENE |
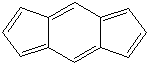
s-INDACENE |
|
|
|
|
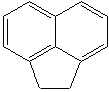
ACENAPHTHALENE (CAS RN: 83-32-9) |
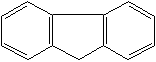
FLUORENE (CAS RN: 86-73-7) |
|
|
|
|
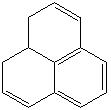
PHENALENE (CAS RN: 203-80-5) |
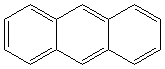
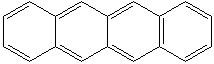
ANTHRACENE (CAS RN:120-12-7) |
|
|
|
|
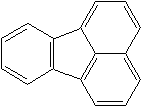
FLUORANTHENE (CAS RN: 206-44-0) |
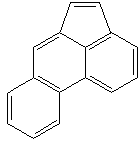
ACEPHENANTHRYLENE |
|
|
|
|
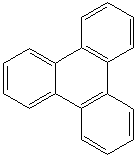
TRIPHENYLENE (CAS RN: 217-59-4) |
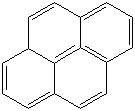
PYRENE (CAS RN: 129-00-0) |
|
|
|
|
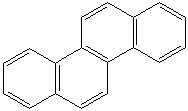
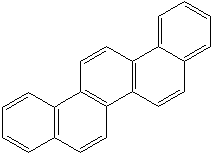
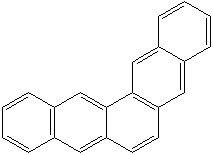
CHRYSENE (CAS RN: 218-01-9) |
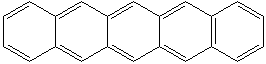
NAPHTHACENE (CAS RN: 92-24-0) |
|
|
|
|
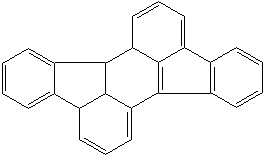
PLEIADENE (CAS RN: ) |
PICENE (CAS RN: 213-46-7) |
|
|
|
|
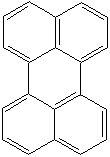
PERYLENE (CAS RN: 198-55-0) |
PENTAPHENE (CAS RN: 222-93-5) |
|
|
|
|
PENTACENE (CAS RN: 135-48-8) |
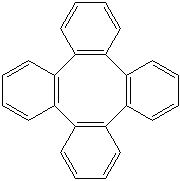
TETRAPHENYLENE (CAS RN: 212-74-8) |
|
|
|
|
RUBICENE (CAS RN: 197-61-5) |
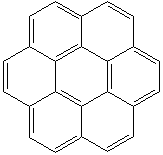
CORONENE (CAS RN: 191-07-1) |
|
|
|
|
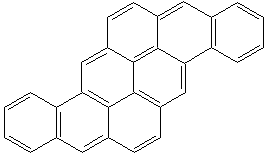
PYRANTHRENE (CAS RN: 191-13-9) |
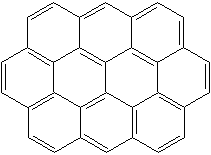
OVALENE (CAS RN:190-26-1) |