PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
220 C
GENERAL DESCRIPTION & APPLICATIONS
APPEARANCE
97.0% min
GENERAL DESCRIPTION OF INDOLE
|
|
|
| ISATIN | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 91-56-5 |
|
| EINECS NO. | 202-077-8 | |
| FORMULA | C8H5NO2 | |
| MOL WT. | 147.13 | |
| H.S. CODE | 2933.79 | |
| TOXICITY | ||
| SYNONYMS | Indole-2,3-dione; 2,3-Indolinedione; 1H-Indole-2,3-dione; | |
| o-Aminobenzoylformic Anhydride; 2,3-Diketoindoline; 2,3-Dioxoindoline; Isatic Acid Lactam; 2,3-Ketoindoline; Isatinic Acid Anhydride; 靛红; | ||
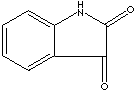
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | yellow to red needle crystalline solid | |
| MELTING POINT | 198 - 204 C (Decomposes) | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Slightly soluble | |
|
SOLVENT SOLUBILITY |
||
| AUTOIGNITION | ||
| pH | ||
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
220 C |
|
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Isatin, an indole derivative, is a yellow to red needle crystalline solid; soluble in hot water. It is a hetero bicyclic aromatic compound with diketone at 2 and 3 positions. Isatin class compounds are mainly used as raw material in dye manufacturing (artificial indigo, disperse dye yellows). It has lactam (a cyclic amide) structure which is an important part of antibiotics, such as penicillin. Cyclic ester structures are active nucleuses in pharmacological activity and flavorings. They are used as intermediates for the synthesis of pharmaceuticals, herbicides, and other chemical compounds. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellow to reddish crystalline solid | |
| ASSAY (G.C) |
97.0% min |
|
| MELTING POINT | 198 - 204 | |
| TRANSPORTATION | ||
| PACKING | 25kgs in drum | |
| HAZARD CLASS | not regulated | |
| UN NO. |
|
|
| OTHER INFORMATION | ||
| Hazard Symbols: XN, Risk Phrases: 22, Safety Phrases: 24/25 | ||
|
GENERAL DESCRIPTION OF INDOLE |
||
| Indole, also called Benzopyrrole, is a yellow crystalline powder with unpleasant aroma. It has the pyrrole ring (five-membered unsaturated ring structure composed of four carbon atoms and one nitrogen atom) which is fused benzene ring. There are tautomerer called indolenine (unsubstituted 3H-indole) and structural isomer, isoindole. But they are unstable. Indole occurs in some plants or in coal tar, and is formed in the intestine during putrefaction and by certain cultures of bacteria. it is commercially synthesized from phenylhydrazine and pyruvic acid. It is used in perfumery and in preparing tryptophan, one of the 20 amino acids commonly found in animal proteins. It has important application in the industry of plant growth. It is used to prepare indoleacetic acid (auxin) and other plant growth substances which help the development of roots in plant. It is used to make selective herbicides. Indole and its derivatives are widely used in making perfumes, agrochemicals and medicines. Indoline, dihydroindole, is used in making agrochemicals and medicines. | ||
|
|
|