|
BUPRENORPHINE HCl |
|
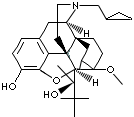
Buprenorphine Hydrochloride; (5alpha,7alpha(S))-alpha-tert-Butyl-17-(cyclopropylmethyl)- 4,5-epoxy-18,19-dihydro-3-hydroxy- 6-methoxy-alpha-methyl- 6,14-ethenomorphinan-7-methanol hydrochloride; 21-Cyclopropyl-7alpha-((S)-1-hydroxy-1,2,2-trimethylpropyl)-6,14-endo-ethano-6,7,8,14- tetrahydrooripavine hydrochloride; Buprenex; Finibron; Subutex; Temgesic; Buprenorfine Hydrochloride; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
52485-79-7 (parent), 53152-21-9 (hydrochloride) |
|
EINECS RN |
257-950-6 (parent), 258-396-8 (hydrochloride) |
|
FORMULA |
C29H41NO4·HCl |
|
MOLE WEIGHT |
504.10 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white or almost white crystalline powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
|
pH |
weakly acidic |
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents. |
| DECOMPOSITION PRODUCTS |
Carbon oxides. nitrogen oxides, Hydrogen chloride gas |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 0, Reactivity: 0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Central nervous system Effect, Harmful by ingestion., Teratogen, Reproductive hazard. |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
Harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
XN |
|
RISK PHRASES |
22 |
|
SAFETY PHRASES |
|
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | |||||||||||||||||||||||||||||||||||||||||
|
Buprenorphine, when appropriately prescribed and taken, is an effective, safe medication approved by the FDA for use in the treatment of opioid addiction. Buprenorphine relieves withdrawal, reduces craving and blocks the effects of heroin in ways similar to methadone.Maintenance doses are generally between 12 and 32 milligrams but (like methadone) should be individualized. Unlike methadone, buprenorphine may be prescribed for treatment of opioid addiction by any doctor who has received training (available via the Internet or as a oneday course) and a waiver from the DEA. This is its principal advantage over methadone for most doctors and patients. Misuse of buprenorphine is less likely than methadone to result in death. (http://www.drugpolicy.org/) Buprenorphine: A prescription medication for people addicted to heroin or other opiates that acts by relieving the symptoms of opiate withdrawal such as agitation, nausea and insomnia. Buprenorphine is more weakly addictive and has a lower risk of overdose than methadone. The effects last for about three days. Buprenorphine is sold under the brand name of Subutex and, in combination with naloxone, as Suboxone. Subutex is intended for use at the beginning of treatment while Suboxone is intended for the maintenance treatment of opiate addiction. (Naloxone was added to guard against intravenous abuse of buprenorphine by individuals physically dependent on opiates.) (http://www.medterms.com/) Drug control under USDEA Schedule III; Home Office Schedule 3; psychotrope; kontrollierte Droge in Deutschland.
|
|||||||||||||||||||||||||||||||||||||||||
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white or almost white crystalline powder |
|
IDENTIFICATION |
passes Test IR, Test B |
|
ASSAY |
98.5 - 101.0 % (on the dried basis) |
|
CLARITY OF SOLUTION |
clear and colorless |
|
LOSS ON DRYING |
1.0% max |
|
SPECIFIC ROTATION |
-92° ~ -98° |
|
PH |
4.0 - 6.0 |
|
WATER |
1.0% max (KF) |
|
RESIDUE ON IGNITION |
0.1% max |
|
RELATED SUBSTANCES |
0.65% max (Total), 0.15% max (Individuals) |
|
SOLVENT RESIDUES |
0.5% max |
|
|
| PRICE |
|
|