|
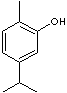
CARVACROL |
| 1-Hydroxy-2-methyl-5-isopropylbenzene; 1-Methyl-2-hydroxy-4-isopropylbenzene; 2-Hydroxy-p-cymene; 2-Methyl-5-(1-methylethyl)phenol; 2-Methyl-5-isopropylphenol; 2-p-Cymenol; p-Cymen-2-ol; 3-Isopropyl-6-methylphenol; 5-Isopropyl-2-methylphenol; 5-Isopropyl-o-cresol; o-Thymol; Antioxine; 6-Methyl-3-isopropylphenol; Isopropyl-o-cresol; Karvakrol; FEMA No. 2245; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
499-75-2 |
|
EINECS RN |
207-889-6 |
|
FORMULA |
(CH3)2CHC6H3(CH3)OH |
|
MOLE WEIGHT |
150.22 |
|
SMILES |
c1(cc(c(C)cc1)O)C(C)C |
|
H.S.CODE |
2907.19.1000 |
|
CLASSIFICATION |
Fungicide, Bactericide, Preservative, Flavors and Fragrances |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
Colorless to yellow liquid |
|
MELTING POINT |
1 C |
|
BOILING POINT |
236 - 238 C |
|
DENSITY |
0.976 |
|
SOLUBILITY IN WATER |
1250 mg/l |
|
pH |
|
| log Pow | 3.49 (Octanol-water) |
| OH RATE | 9.95E-11 (cm3/molecule-sec at 25 C Atmospheric) |
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
1.523 |
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents, Strong bases. |
| DECOMPOSITION PRODUCTS | carbon oxides |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 3; Flammability: 1; Reactivity: 0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Corrosive. Harmful if swallowed. Causes burns. |
|
EYE |
Causes burns |
|
SKIN |
Causes burns |
|
INGESTION |
Harmful if swallowed. |
|
INHALATION |
Material is extremely destructive to the tissue of the mucous membranes and upper respiratory tract. May be harmful if inhaled. |
| TOXICITY | Rat LD50 (Oral): 810mg/kg BEHAVIORAL: somnolence (general depressed activity), convulsions or effect on seizure threshold |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
3265 |
| HAZARD CLASS |
8 |
| PACKING GROUP |
III |
| HAZARD SYMBOL |
C |
|
RISK PHRASES |
22-34 |
|
SAFETY PHRASES |
26-36/37/39-45 |
|
|
| GENERAL DESCRIPTION & EXTERNAL LINKS |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
(http://www.henriettesherbal.com/) The effects of carvacrol, a natural biocide, on dual-species biofilms formed by Staphylococcus aureus and Salmonella enterica serovar Typhimurium were investigated with a constant-depth film fermentor. Biofilm development reached a quasi-steady state in 12 days at 25°C with S. aureus predominance (~99%). Cryosectional analysis detected viable S. aureus and S. enterica serovar Typhimurium at depths of 320 and 180 µm from the film surface, respectively. Carvacrol pulses (1.0 mmol/h) inhibited S. aureus by 2.5 log CFU/biofilm during the early stages of film formation, ultimately causing a significant reduction (P<0.001) of the staphylococcal population at quasi-steady state. Initial carvacrol pulsing elicited a 3 log CFU/biofilm reduction in viable S. enterica serovar Typhimurium, and additional periodic carvacrol pulses instigated significant inhibition of salmonellae (1 to 2 log CFU/biofilm) during biofilm development. Carvacrol pulsing reduced protein levels fivefold (P<0.001) during initial biofilm development. Comparative studies with a peroxide-based commercial sanitizer (Spor-Klenz RTU) revealed that this commercial sanitizer was more biocidal than carvacrol during early biofilm development. When the biofilm reached quasi-steady state, however, periodic pulses with 1 mmol of carvacrol per h (P=0.021) elicited a significantly higher inhibition than Spor-Klenz RTU (P=0.772). Dual-species microcolonies formed under the influence of continuously fed low carvacrol concentrations (1.0 mmol/h) but failed to develop into a mature quasi-steady-state biofilm and did not reach any stage of film formation in the presence of high concentrations (5.0 mmol/h). These data show that carvacrol is an effective natural intervention to control dual-species biofilm formation.
Carvacrol is an
isomer of thymol. It is used as a flavor ingredient and also as an
antibacterial or antifungal agent. Thymol products include:
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
Colorless to yellow liquid |
|
ASSAY |
98.0% max |
|
BOILING POINT |
236 - 238 C |