|
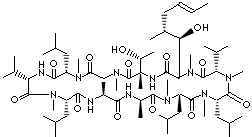
CYCLOSPORIN C |
| 5S,8S,11S,14R,17S,20S,23S,26S,32S)-32-[(1R)-1-hydroxyethyl]-2-[(E,1R,2R)-1-hydroxy-2-methylhex-4-enyl]- 3,6,9,12,14,17,21,27,30-nonamethyl-8, 11,20,26-tetrakis(2- methylpropyl)- 5,23-di(propan-2-yl)-3,6,9, 12,15,18, 21,24,27,30,33-undecazacyclotritriacontane-1,4,7,10,13,16,19,22,25,28, 31-undecone; Cyclo(L-alanyl- D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4- dimethyl-L- 2-amino- 6-octenoyl-L-threonyl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl); [Thr2]CsA; 7-Threonine-cyclosporin A; [Thr2-Cyclosporin A]; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
59787-61-0 |
|
EINECS RN |
|
|
FORMULA |
C62H111N11O13 |
|
MOLE WEIGHT |
1218.61 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white crystalline powder |
|
MELTING POINT |
152 - 155 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble (soluble in ethanol, organic solvents) |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong acids, Alkali metals, Aluminum, |
| DECOMPOSITION PRODUCTS |
Carbon oxides. Nitrogen oxides. |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 0, Reactivity: 0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Target Organ Effect, Harmful by ingestion., Carcinogen. Target Organs: Immune system., Kidney, Liver, Blood. |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
Harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD CODE |
T |
|
RISK STATEMENTS |
45-60-22 |
|
SAFETY STATEMENTS |
53-45 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cyclosporin A (CAS No. 59865-13-3) is a strong immunosuppressant agent (Laupacis et al., 1982; Wagner, 1983) used for the treatment of kidney, liver, heart, and other organ transplantation; rheumatoid arthritis; and psoriasis (Faulds et al., 1993). Cyclosporin A is a non-polar cyclic polypeptide consisting of 11 amino acids produced by multiple fungal species (Petcher et al., 1976; Krensky et al., 2006; Budavari et al., 1996). Cyclosporin A is a white to off white crystalline solid that is slightly soluble in water and soluble in organic solvents (Budavari et al., 1996). (http://www.vcu.edu/) Cyclosporin (brand names Sandimmun and Neoral) is a medicine used to treat rheumatoid arthritis and other rheumatic conditions such as systemic lupus erythematosus (lupus/SLE) and polymyositis (muscle inflammation). Cyclosporin is used in rheumatoid arthritis when other treatments have been unsuccessful. It is also used to prevent the rejection of transplanted organs. Cyclosporin is an immunosuppressive medicine, which means that it works by reducing the activity of the immune system. In rheumatoid arthritis, this action helps to reduce inflammation and thus reduce pain and swelling. It also limits damage to the joints and helps to prevent disability in the long term. Because cyclosporin reduces the damage to the joints, rather than just relieving the pain, it belongs to the group of medicines called disease modifying antirheumatic drugs (DMARDS). (http://www.rheumatology.org.au/) Discovery of immunosuppression by cyclosporin in 1976 is attributed to J. F. Borel, see Figure 5. In 1983 cyclosporin was approved for clinical use to prevent graft rejection in transplantation. Most of the surgical problems of allograft transplantation had already been solved by this time. Since 1961 the standard method of achieving immunosuppression had been a combination of azathioprine and corticosteroids. Azathioprine inhibits cell proliferation non-selectively. Its main unwanted side effect is depression of the bone marrow, other toxic effects include increased susceptibility to infections, a mild hepatotoxicity, skin eruptions, nausea and vomiting. Corticosteroids inhibit T lymphocytes and have an anti-inflammatory effect. Side effects include diabetes, avascular necrosis of bones and increased tendency to infections. Cyclosporin was the strongest immunosuppressor to be discovered so far, it also overcame many of the risk factors associated with azathioprine and is relatively non-toxic to bone marrow. With the introduction of cyclosporin patient morbidity fell. It became possible to transplant organs with a one year success of 20% higher than previously, and to transplant organs successfully which previously had only been done in experimentation: the heart, the liver, the lung and combined heart lung transplants. As well as transplantation, cyclosporin has been used in most autoimmune diseases. In the 1980’s experimental treatment with cyclosporin of insulin-dependent diabetes mellitus, inflammatory bowel disease, chronic asthma, atopic dermatitis, aplastic anaemia and psoriasis supported evidence of their T cell mediated nature. (http://www.world-of-fungi.org/)Cyclosporins
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white crystalline powder |
|
ASSAY |
98.5% - 101.5% |
|
LOSS ON DRYING |
2.0% max |
|
HEAVY METALS |
10ppm max |
|
MELTING POINT |
152 - 155 C |
|
|
| PRICE INFORMATION |
|
|