|
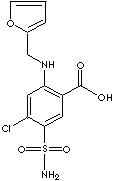
FUROSEMIDE |
| 2-Furfurylamino-4-chloro-5-sulfamoylbenzoic acid; 4-Chloro-5-sulfamoyl-N-furfuryl-anthranilic acid; 4-Chloro-N-(2-furylmethyl)-5-sulfamoylanthranilic acid; 4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid; 5-(Aminosulfonyl)-4-chloro-2-((2-furanylmethyl)amino)benzoic acid; Aisemide; Chlor-N-(2-furylmethyl)-5-sulfamylanthranilsaeure; Frusemide: Fursemide: |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
54-31-9; 41733-55-5 (hydrochloride) |
|
EINECS RN |
200-203-6 |
|
FORMULA |
C12H11ClN2O5S |
|
MOLE WEIGHT |
330.74 |
|
CHEMICAL FAMILY |
benzoic-sulfonamide-furan |
| RELATED CATEGORIES |
Loop diuretic; antihypertensive / Sodium Potassium Chloride Symporter Inhibitors |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
White to slightly yellow crystalline powder; odorless. |
|
MELTING POINT |
203 - 206 C (Decomposes) |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble (Insoluble in dichloromethane; slightly soluble in chloroform; sparingly soluble in alcohol; slightly soluble in ether; soluble in methanol; freely soluble in acetone, in dimethylformamide, disolves in dilute solution of alkali hydroxides) |
|
pH |
5 (aqueous solution) |
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides, nitrogen oxides (NOx), Sulfur oxides, Hydrogen chloride gas |
| POLYMERIZATION |
will nor occur |
|
TOXICITY |
Oral Rat: LD50: 2600 mg/kg, Oral Mouse: LD50: 2200 mg/kg |
|
|
| SAFETY |
|
|
HAZARD NOTES |
This substance is not classified as dangerous |
|
EYE |
May cause eye irritation. In case of eye contact, flush eyes with water as a precaution. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. In case of skin contact, wash off with soap and plenty of water. |
|
INGESTION |
May be harmful if swallowed. Never give anything by mouth to an unconscious person. Rinse mouth with water. |
|
INHALATION |
May cause irritation. May cause respiratory tract irritation. If breathed in, move person into fresh air. |
|
TARGET ORGANS |
Liver, Kidney, ears, Male reproductive system. |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
T |
|
RISK PHRASES |
61 |
|
SAFETY PHRASES |
22-36/37-53-45-53 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
|
Wikipedia Linking: http://en.wikipedia.org/wiki/Furosemide Furosemide is one of the most effective & least toxic diuretics used in pediatric practice. Experimental & clinical data suggest that adrenocorticosteroids &/or endogenous ouabain-like substances may play an important role in its diuretic effect. Also, the drug appears to have anti-inflammatory properties. In children with different diseases who received orally or iv 1-2 mg/kg doses of furosemide, a statistically significant positive linear relationship was found between the drug urinary excretion rate & the urine flow rate, but log dose-response curves to the drug were found to vary depending on the disease & the route of the drug admin. No sigmoid-shaped log dose-response curve (ie, one approaching a zero response at very low furosemide urinary excretion rates & a max response at very high excretion rates) was attained, which may suggest that the capacity of the kidney tubules to respond diuretically to the aforementioned doses of furosemide was not exceeded in these patients. The pharmacologic effects of furosemide are similar to those of ethacrynic acid. The exact mode of action of furosemide has not been clearly defined; in contrast to ethacrynic acid, it does not bind sulfhydryl groups of renal cellular proteins. Furosemide inhibits the reabsorption of electrolytes in the ascending limb of the loop of Henle. The drug also decreases reabsorption of sodium and chloride and increases potassium excretion in the distal renal tubule and exerts a direct effect on electrolyte transport at the proximal tubule. Furosemide does not inhibit carbonic anhydrase and is not an aldosterone antagonist. (http://toxnet.nlm.nih.gov/)In the presence of ouabain both ethacrynic acid and furosemide exerted similar effects on sodium outflux, inhibiting approximately 0.5 mmoles/L of cells per hr. This component of sodium outflux has been called outflux-fraction II. Ethacrynic acid showed no inhibitory potency when ouabain and furosemide were present, thereby suggesting that the same outflux component (fraction II) was affected by ethacrynic acid and by furosemide. In addition, furosemide reduced sodium influx to the same extent that it reduced sodium outflux. Outflux-fraction II, as defined by furosemide, did not contribute a net sodium outflux. These results of sodium outflux and influx experiments confirm the existence of a transport pathway which does not contribute to net flux and which fits the definition of exchange diffusion. (http://www.jci.org/) The physiological effects of furosemide, a new diuretic agent chemically related to thiazide diuretics, have been evaluated in seven normal subjects and in 39 patients with edema of varied origin. The compound exhibited an unusually broad dose-response curve so that increasing diuresis could be induced with oral doses of from 40 mg once daily to 600 mg three times daily. At the higher dosages furosemide was significantly more effective than conventional thiazide diuretics and exhibited an order of potency which can be achieved with ethacrynic acid. In many of its diuretic properties furosemide resembled thiazide agents. The natriuresis and diuresis which it produced was associated with a disproportionate loss of chloride and potassium and the consequent production of degrees of hypokalemic alkalosis. (http://circ.ahajournals.org/)
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to off-white crystalline powder |
|
IDENTIFICATION |
Pass Test IR, UV |
|
ASSAY |
98.5 - 101.0% |
| HEAVY METALS | 20ppm max |
|
SULFATES |
300ppm max |
|
CHLORIDE |
200ppm max |
|
SULFATED ASH |
0.1% max |
| LOSS ON DRYING |
0.5% max |
|
RELATED SUBSTANCES |
Individual
impurity: 0.1% max |
|
MICROBIOLOGY |
Total
Bacterial counts: 100CFU/g max |
| RESIDUAL SOLVENTS | Methylene Chloride: 600ppm max |
|
|
| PRICE |