|
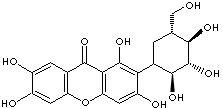
MANGIFERIN |
| 2-beta-D-Glucopyranosyl-1,3,6,7-tetrahydroxy-9H-xanthen-9-one; Alpizarin; Alpizarine; Aphloiol; Chinomin; Chinonin; 1,3,6,7-Tetrahydroxy-2-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2- yl)xanthen-9-one; ,3,6,7-Tetrahydroxyxanthone C2-beta-D-glucoside; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
4773-96-0, 99331-60-9 |
|
EINECS RN |
|
|
FORMULA |
C19H18O11 |
|
MOLE WEIGHT |
422.34 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
yellow crystalline powder |
|
MELTING POINT |
274 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 4; Flammability: 0; Reactivity:0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Highly Toxic. Very toxic if swallowed. Target organs: Immune system. |
|
EYE |
|
|
SKIN |
|
|
INGESTION |
|
|
INHALATION |
|
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
3462 |
| HAZARD CLASS |
6.1 |
| PACKING GROUP |
I |
| HAZARD SYMBOL |
T+ |
|
RISK PHRASES |
28 |
|
SAFETY PHRASES |
22-28-36/37-45 |
|
|
| OTHER INFORMATION |
||||||||||||||||||||||||||||||
Xanthene is a
polyaromatic cyclic ether compound which have one oxygen in the ring system.
Two benzene rings are fused to tetrahydropyran which is a six membered cyclic
ether with five carbons and one oxygen. It is soluble alcohol and ether. Pyran
ring structure is the basis of the pyranoses. Xanthene is used as a fungicide
itself. But the main function is as the fundamental structure of chromophore
particularly luminescent dyes. Xanthene class dyes include fluorenes, pyronins,
succineins, sacchareins, rosamines, rhodamines, rhodols and fluorones. Xanthone
is the xanthene ketone. It is used as an ovicide and as a larvicide. The
structure of xanthone is found in natural constituents of plants (specially
in mangosteen fruits) and micro-organisms. Naturally occurring xanthones
are under research in activating biological properties for antioxidant and various
pharmaceutical activities including anti-inflammatory,
anticancer, and central
nervous system depressants. Synthetic xanthone can be prepared from
phenyl
salicylate. Synthetic hydroxyxanthones are used as a plastic additive for the
resistance to degradation by light and UV. (Xanthine
is not a xanthene structure compound but dioxopurine, a precursor of uric acid).
Some example of hydroxyxanthone are:
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
yellow crystalline powder |
|
ASSAY |
95.0% max |
|
HEAVY METALS |
20ppm max |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |