|
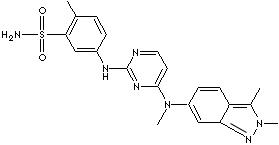
PAZOPANIB |
| 5-((4-((2,3-dimethyl-2H-indazol-6-yl)methylamino)-2-pyrimidinyl)amino)-2-methylbenzenesulfonamide; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
444731-52-6, 790713-33-6, 635702-64-6 (HCl) |
|
EINECS RN |
|
|
FORMULA |
C21H23N7O2S |
|
MOLE WEIGHT |
437.52 |
|
CLASSIFICATION |
Tyrosine Kinase Inhibitor |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon monoxide, Carbon dioxide, Nitrogen oxides, Hydrogen chloride |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health:1 , Flammability:0 , Reactivity:1 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
Harmful if swallowed. |
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
Harmful if swallowed. |
|
INHALATION |
Harmful if inhaled. Material may be irritating to mucous membranes and respiratory tract. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
XN |
|
RISK PHRASES |
22 |
|
SAFETY PHRASES |
22 |
|
|
| EXTERNAL LINKS & GENERAL INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pazopanib hydrochloride (GW786034) is an oral multi-targeted tyrosine kinase receptor inhibitor with anti-tumour activity. Pazopanib inhibits vascular endothelial growth factor receptor (VEGFR) -1, -2 and -3, platelet-derived growth factor receptor (PDGFR), and ckit, which may result in inhibition of angiogenesis in tumours in which these receptors are upregulated. Pazopanib is administered at 800mg once daily as monotherapy. Pazopanib hydrochloride is also in phase II/III development for breast cancer, and in phase II trials in patients with gynaecological cancer, non-small cell lung cancer, peritoneal cancer, nasopharyngeal cancer, pancreatic cancer, soft tissue sarcoma, and glioma. Pazopanib may have advantages over oral multi-targeted tyrosine kinase receptor inhibitors currently licensed (sunitinib and sorafenib) in improving efficacy and/or tolerability in patients with metastatic RCC. (http://www.pcpoh.bham.ac.uk/) It’s certainly important to continue the search for new kidney cancer treatments. Surgery is an effective treatment for some patients with early-stage disease, but many others will have a recurrence (return of cancer) after surgery. When first diagnosed, many patients already have advanced kidney cancer. And because kidney cancer generally resists standard chemotherapy, new treatments are needed. Researchers are studying a new drug called pazopanib for use in many different tumor types, including kidney and ovarian cancers. A targeted treatment, pazopanib helps stop cancer by blocking substances called growth factors that promote the growth and division of tumor cells. People with advanced kidney cancer who had not received any treatment or had not responded to an earlier treatment took part in a clinical trial. For 12 weeks, they took pazopanib once a day. Early findings showed that tumors shrank in about 25 percent of patients; in about 45 percent of the patients, the tumors neither shrank nor grew. (http://www.cancercare.org/)
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white powder |
|
IDENTIFICATION |
Conforms NMR, HPLC, MS |
|
ASSAY |
98.5 - 100.5% |
|
HEAVY METALS |
10ppm max |
|
RESIDUE ON IGNITION |
0.15% max |
|
LOSS ON DRYING |
0.5% max |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|