|
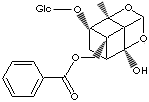
PEONIFLORIN |
| Paeonia moutan; Paeony root; ((1aS,2R,3aR,5R,5aR,5bS)-1a-(b-D-glucopyranosyloxy)-5-hydroxy- 2-methyltetrahydro -1H-2,5-methano-3,4-dioxacyclobuta(cd)pentalen-5b(3aH)-yl)methyl benzoate; 5beta-((Benzoyloxy)methyl)tetrahydro-5-hydroxy-2-methyl-2,5-methano-1H- 3,4-dioxacyclobuta(cd) pentalen-1alpha(2H)-yl-beta-D-glucopyranoside |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
23180-57-6 |
|
EINECS RN |
245-476-2 |
|
FORMULA |
C23H28O11 |
|
MOLE WEIGHT |
480.46 |
|
CHEMICAL FAMILY |
Terpene |
| CATEGORIES | Extractives and their physically modified derivatives |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
white powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Soluble (soluble in methanol, ethanol) |
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBILITIES |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides |
| POLYMERIZATION | |
|
TOXICOLOGICAL |
|
|
|
| SAFETY |
|
|
HAZARD NOTES |
|
|
EYE |
May cause eye irritation. |
|
SKIN |
May cause skin irritation. May be harmful if absorbed through skin. |
|
INGESTION |
May cause irritation. May be harmful if swallowed. |
|
INHALATION |
May cause irritation. May be harmful if inhaled. May cause respiratory tract irritation. |
|
TARGET ORGANS |
|
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
|
|
|
| EXTERNAL LINKS & GENERAL INFORMATION |
|
Heat shock proteins (HSPs) are induced by various physical, chemical, and biological stresses. HSPs are known to function as molecular chaperones, and they not only regulate various processes of protein biogenesis but also function as lifeguards against proteotoxic stresses. Because it is very useful to discover nontoxic chaperone-inducing compounds, we searched for them in herbal medicines. Some herbal medicines had positive effects on the induction of HSPs (Hsp70, Hsp40, and Hsp27) in cultured mammalian cells. We next examined 2 major constituents of these herbal medicines, glycyrrhizin and paeoniflorin, with previously defined chemical structures. Glycyrrhizin had an enhancing effect on the HSP induction by heat shock but could not induce HSPs by itself. In contrast, paeoniflorin had not only an enhancing effect but also an inducing effect by itself on HSP expression. Thus, paeoniflorin might be termed a chaperone inducer and glycyrrhizin a chaperone coinducer. Treatment of cells with paeoniflorin but not glycyrrhizin resulted in enhanced phosphorylation and acquisition of the deoxyribonucleic acid–binding ability of heat shock transcription factor 1 (HSF1), as well as the formation of characteristic HSF1 granules in the nucleus, suggesting that the induction of HSPs by paeoniflorin is mediated by the activation of HSF1. Also, thermotolerance was induced by treatment with paeoniflorin but not glycyrrhizin. Paeoniflorin had no toxic effect at concentrations as high as 80 μg/ mL (166.4 μM). To our knowledge, this is the first report on the induction of HSPs by herbal medicines. (http://www.ncbi.nlm.nih.gov/) Paeoniflorin is a bioactive monoterpene glucoside presenting in the root of Paeonia lactiflora Pall. (family Ranunculaceae), which has been widely used to treat inflammation and arthritic conditions according to the traditional Chinese medical system. The therapeutic effects of the herb and its active component, paeoniflorin, have been confirmed by experimental pharmacological investigations (Takagi and Harada, 1969a,b). However, previous pharmacokinetic studies have shown that paeoniflorin has a poor absorption rate, and thus a very low bioavailability (3-4%) when administered orally. This is probably due to limited transportation of paeoniflorin across the gastrointestinal mucosa (Takeda et al., 1995, 1997). In Mainland China, the total glucosides of Paeonia lactiflora Pall. (TGP) comprising more than 70% of paeoniflorin, has been approved by the State Food and Drugs Administration of China for the clinical application in treating rheumatic and arthritic diseases as a patented botanical drug (Zhao et al., 1997). While the drug is useful, nevertheless, the low bioavailability of paeoniflorin might be restricting its therapeutic efficacy. Therefore, this investigation aims to use the pharmacokinetic profile of paeoniflorin to find a way to improve its bioavailability and thereby enhance its therapeutic efficacy.... (http://wlv.openrepository.com/)
|
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white powder |
| CONTENT |
98.0% min |
| LOSS ON DRYING |
1.0% max |
|
RESIDUE ON IGNITION |
0.5% max |
|
RESIDUAL SOLVENTS |
EU2000 |
|
HEAVY METALS |
20ppm max |
|
ARSENIC |
2ppm max |
|
PARTICLE SIZE |
80mesh (100%) |
| MICROBIOLOGICAL TESTS |
Total Plate Count: 1000 CFU/g max |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |