|
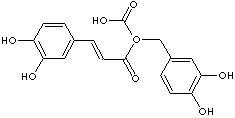
ROSMARINIC ACID |
| alpha-(((3,4-Dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-3,4-dihydroxy- benzenepropanoic acid; 3,4-Dihydroxycinnamic acid 2-ester with 3-(3,4-dihydroxyphenyl)lactic acid; R-(+)-2-(3,4-Dihydroxycinnamoyloxy)-3-(3,4-dihydroxyphenyl)propionic acid; Rosemary acid; (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid; 3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester; (2R)-3-(3,4-Dihydroxyphenyl)-2-[(E)- 3-(3,4-dihydroxyphenyl) prop- 2-enoyl]oxypropanoic acid; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
20283-92-5 |
|
EINECS RN |
|
|
FORMULA |
C18H16O8 |
|
MOLE WEIGHT |
360.31 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
red to brown powder |
|
MELTING POINT |
171 - 174 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
|
pH |
|
|
VAPOR DENSITY |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides |
| POLYMERIZATION | Has not been reported |
|
NFPA RATINGS |
Health: 4; Flammability: 0; Reactivity:0 |
|
|
| SAFETY |
|
|
HAZARD NOTES |
|
|
EYE |
May cause eye irritation. |
|
SKIN |
May be harmful if absorbed through skin. May cause skin irritation. |
|
INGESTION |
May be harmful if swallowed. |
|
INHALATION |
May be harmful if inhaled. May cause respiratory tract irritation. |
|
CHRONIC |
|
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP |
|
| HAZARD SYMBOL |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
|
|
|
| OTHER INFORMATION |
|
Rosmarinic acid, together with similar compounds, has been known as „Labiatengerbstoff“ even before its chemical structure was elucidated. Indeed, it is a tannin-like compound, sometimes described as a depside of caffeic acid. Originally identified in rosemary (Rosmarinus officinalis L.), the structure was elucidated as an ester of caffeic acid and 3-(3,4-dihydroxyphenyl)lactic acid. The compound has been reported to occur in several taxonomically non-related families of the plant kingdom. It has attracted much attention since it was identified to be the main compound responsible for the antiviral activity of lemon balm in treating Herpes simplex. http://www.fpharm.uniba.sk/ Peroxynitrite (ONOO−) is considered to be more toxic to cell than either of its precursors, NO or .O2−, because ONOO− is a powerful oxidant that can cause damages of proteins, lipids, and DNA through more subtle action mechanism, which is called the process of nitration and oxidation. Because of these potential effects, it is important to study the exact nature of cytotoxic property of ONOO− scavengers which should be supplemented. In the present study, we investigated reactive oxygen species (ROS)/reactive nitrogen species (RNS) scavenging activities for rosmarinic acid. Rosmarinic acid showed evidence of scavenger for ONOO−. In lipopolysaccharide(LPS)-treated spleen and kidney, rosmarinic acid decreased the ROS and ONOO− levels. The data from the experiments confirmed a protective effect of rosmarinic acid on ROS generation in vascular endothelial cells. Further, rosmarinic acid was found to block tert-butyl hydroperoxide (t-BHP)-mediated ROS generation. The present study demonstrated that rosmarinic acid exerts an anti-oxidative effect by scavenging ONOO−. http://korea-biogerontology.org/ |
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
red to brown powder |
|
ASSAY |
95.0% max |
|
HEAVY METALS |
20ppm max |