|
AMOROLFINE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 78613-35-1 |
|
| EINECS NO. | ||
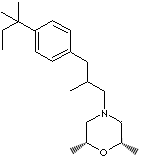
| FORMULA | C21H35NO | |
| MOL WT. | 317.51 | |
|
H.S. CODE |
2934.99.9000 | |
|
TOXICITY |
|
|
| SYNONYMS | Amorolfina; Amorolfinum; | |
| cis-2,6-Dimethyl-4-(2-methyl-3-(p-tert-pentylphenyl)propyl)morpholine; (2R,6S)-2,6-Dimethyl-4-(2- methyl-3-(4-(2-methylbutan-2-yl)phenyl)propyl)morpholine | ||
|
SMILES |
C1[C@H](O[C@H](CN1C[C@@H](Cc1ccc(cc1)C(CC)(C)C)C)C)C | |
CLASSIFICATION |
Antimycotic, Antifungal |
|
|
EXTRA NOTES |
ATC code D01AE16. For preparations for topical and systemic treatment of dermatological mycoses. Preparations with systemic antimycotic effect |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white to off-white crystalline powder | |
| MELTING POINT |
|
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
|
|
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
|
|
| FLASH POINT |
|
|
| STABILITY |
Stable under ordinary conditions. |
|
|
GENERAL DESCRIPTION AND EXTERNAL LINKS |
||
|
AMOROLFINE is a dimethylmorpholine antifungal agent for topical use as a cream, lacquer, and ethanol-based spray. Vaginal formulations (ovules, tablets) have also been investigated. AMOROLFINE is unrelated to imidazoles, allylamines or polyene antibiotics. Antifungal effects of AMOROLFINE are secondary to reduced ergosterol synthesis. This is mediated by inhibition of 2 enzymes, delta-14-reductase and delta-7,8-isomerase, the former being more important for antifungal activity. Other actions may also be contributory, including deposition of abnormal chitin with subsequent growth abnormalities.(http://www.bpfk.gov.my/) Amorolfine is a topical antifungal agent used to treat onychomycosis and dermatophytosis. It is prepared as a cream or nail lacquer. Amorolfine is a morpholine derivative that appears to act by interfering with the synthesis of sterols essential for the functioning of fungal cell membranes. In vitro, activity has been shown against some yeasts and dimorphic, dematiaceous, and filamentous fungi ( Blastomyces dermatitidis , Candida spp , Histoplasma capsulatum , Sporothrix schenckii , and Aspergillus spp ). Despite its in vitro activity, amorolfine is inactive when given systemically and thus is limited to topical use in the treatment of superficial infections. Its role in the treatment of fungal infection in animals is not clear.(http://www.merckvetmanual.com/) Amorolfine is a structurally unique, topically active antifungal agent, which possesses both fungistatic and fungicidal activity in vitro. Its spectrum of in vitro activity includes dermatophyte, dimorphic, some dematiaceous and filamentous fungi, and some yeasts. In clinical trials, application of amorolfine 5% nail lacquer once or twice weekly for up to 6 months produced mycological and clinical cure in approximately 40 to 55% of patients with mild onychomycosis 3 months after cessation of therapy. Overall cure and improvement was observed in approximately 85 to 90% of patients with superficial dermatomycoses following treatment with amorolfine 0.25% cream for up to 6 weeks. However, few controlled, comparative trials are available for these different mycoses, and only small numbers of patients have been evaluated to date. Both preparations appear to be well tolerated; only minor localised adverse events have been reported in clinical trials. At present, the major potential indication for topical amorolfine appears to be onychomycosis. Within this clinical setting, amorolfine should be reserved for patients with mild infection without nail matrix involvement. Systemic therapy, however, remains essential for patients with severe intractable onychomycosis involving the nail bed. Evidence to date does not clarify whether the use of adjuvant topical amorolfine reduces the need for systemic therapy in patients with severely infected nails, or whether amorolfine is beneficial in individuals unresponsive to other treatment options. (http://lib.bioinfo.pl/) |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to off-white crystalline powder | |
|
ASSAY |
99.0% min | |
| RELATED SUBSTANCES | Total impurity: 1.0% max, Individual impurity: 0.5% max |
|
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||