PRODUCT IDENTIFICATION
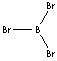
BBr3
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
-46.3 C
91.3 C
SOLUBILITY IN WATER
reacts (soluble in Hydrocarbons, CCl4, CH2Cl2, SiCl2)
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
Boron tribromide, BBr3 is very volatile and reacts vigorously with water in air to form boric acid and hydrogen bromide. It is commercially prepared from boron oxide with carbon generates free boron which reacts vigorously with the bromine. This compound has commercial application as a strong Lewis acid, An electrophile or electron acceptor. Some common Lewis acids include aluminium chloride, iron chloride, boron trifluoride, niobium pentachloride and the lanthanide triflates. In chemical synthesis, Boron Ttribromide is an excellent demethylating or dealkylating agent for ethers and find application as a catalyst polymerization process and in Friedel-Crafts chemistry. Boron tribromide is used as a source of boron in pre-deposition processes for doping in the manufacture of semi-conductors.
APPEARANCE
ASSAY
99.5.0% min
0.5% max
Hazard Symbols: T+ C , Risk Phrases: 14-26/28-35-40, Safety Phrases: 23-26--28A-36/37/39-45-9