|
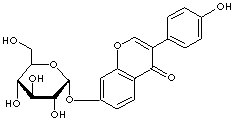
DAIDZIN |
| Synonyms. Daidzin; 7-(beta-D-Glucopyranosyloxy)-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 7-(beta-D-Glucopyranosyloxy)-3-(4-hydroxyphenyl)-4H-chromen-4-one; Daidzein 7-O-glucoside; Daidzein 7-glucoside; Daidzoside; 4',7-Dihydroxyisoflavone; Other RN: 1329-08-4. 28572-56-7 |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
552-66-9 |
|
EINECS RN |
|
|
FORMULA |
C21H20O9 |
|
MOLE WEIGHT |
416.38 |
|
H.S CODE |
2938.90.0000 |
|
SMILES |
c1(=O)c2ccc(cc2occ1c1ccc(cc1)O)O[C@@H]1O[C@@H]([C @@H](O) [C@@H](O)[C@H]1O)CO |
|
CLASSIFICATION |
Alcohol deterrent, Enzyme inhibitor, Isoflavone |
|
EXTRA NOTES |
A potent, selective, and reversible inhibitor of human mitochondrial aldehyde
dehydrogenase Daidzin is an isoflavone reported to have anti-oxidant, anti-carcinogenic, and anti-artherosclerotic activities. Daidzin is the major active component of the traditional Chinese medicine (TCM) derived from radix puerariae (Kudzu) that is commonly used to treat alcohol abuse. Daidzin is a potent and selective inhibitor of mitochondrial aldehyde dehydrogenase 2 (ALDH-2). |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE. |
white powder |
|
MELTING POINT |
234 - 242 C (Decomposes) |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble |
| SOLVENT SOLUBILITY | Soluble in DMSO or dilute aqueous base |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong Oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health hazard: 0, Flammability: 0, Physical hazards: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Drug Information Portal (U.S. National Library of Medicine) - Daidzin PubChem Compound Summary - Daidzin Drug Bank - Daidzin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Daidzin http://www.ebi.ac.uk/ - Daidzin http://www.ncbi.nlm.nih.gov/ - Daidzin http://pubs.acs.org/ http://www.pnas.org/ http://extension.agron.iastate.edu/ |
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white powder |
|
ASSAY |
98.0% min |
| LOSS ON DRYING |
0.5% max |
| MELTING POINT |
236 - 242 C |
| SOURCE | Pueraria lobata (Willd.) Ohwi |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
Not regulated |
| HAZARD SYMBOL |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Not known |
| HAZARD CODES |
|
|
RISK PHRASES |
36/38 |
|
SAFETY PHRASES |
26-36 |
|
|