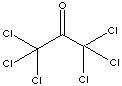
HEXACHLOROACETONE
PRODUCT IDENTIFICATION
116-16-5
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
Clear liquid
208 - 212 C
1.735 - 1.745
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION APPLICATIONS
Hexachloroacetone is a haloketone consisting of two electron withdrawing groups (a ketone group and alpha-halogen substituent). The halogen substituent makes the carbon atom of the carbonyl group more electropositive. Haloketone reacts faster than n-alkylhloride in nucleophilic aliphatic substitution. Haloketones are important in substituted heterocycle compounds synthesis. Hantzsch Pyrrole Synthesis and the Hantzsch thiazole synthesis are examples. Haloketone are reagents for the preparation of regio- and stereoselective vinylic, allylic, or enamine compounds. In the crossed aldol reaction haloketone can be converted to halohydrin which subsequently forms an epoxide in presence of a base.
APPEARANCE
Clear liquid
99.0% min
660C - 70 C at 6mm Hg
250kg in drum
OTHER INFORMATION