|
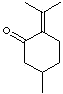
R-PULEGONE |
| Synonyms. (+)-(R)-Pulegone; (+)-Pulegone; (1R)-(+)-p-Menth-4(8)-en-3-one; (R)-(+)-Pulegone; (R)-Pulegone; 1-Isopropylidene-4-methyl-2-cyclohexanone; 1-Methyl-4-isopropylidene-3-cyclohexanone; delta-4(8)-p-Menthen-3-one; 3-Methyl-6-isopropylidenecyclohexanone; (R)-5-Methyl-2-(1-methylethylidene)cyclohexanone; Pulegon; Pulegone; D-Pulegone; (R)-(+)-p-Menth-4(8)-en-3-one; (5R)-5-Methyl-2-(1-methylethylidene)cyclohexanone; (R)-5-Methyl-2-(1-methylethylidene)cyclohexanone; p-Menth-4(8)-en-3-one; Other RN: 90449-51-7 |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
89-82-7 |
|
EINECS RN |
201-943-2 |
|
FORMULA |
C10H16O |
|
MOLE WEIGHT |
152.23 |
|
H.S CODE |
2914.29.5000 |
|
SMILES |
C1(\C(C[C@@H](C)CC1)=O)=C(/C)C |
|
CLASSIFICATION |
Ketone, Perfume ingredient, Monoterpene |
|
EXTRA NOTES |
FEMA No. 2963 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE |
Clear to yellowish liquid with strong mint odor |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
0.932 - 0.938 |
|
SOLUBILITY IN WATER |
Insoluble |
| SOLVENT SOLUBILITY | Miscible with ethanol, chloroform, Ether |
|
VAPOR DENSITY |
5.3 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
1.485 - 1.488 |
|
FLASH POINT |
82 C |
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| DECOMPOSITION PRODUCTS |
Carbon oxides. |
| POLYMERIZATION |
|
|
NFPA RATINGS |
Health Hazard: 1, Fire: 2, Reactivity Hazard: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Drug Information Portal (U.S. National Library of Medicine) - Pulegone PubChem Compound Summary - Pulegone IPCS INCHEM - Pulegone KEGG (Kyoto Encyclopedia of Genes and Genomes) - Pulegone http://www.ebi.ac.uk/chebi/ - Pulegone http://www.ncbi.nlm.nih.gov/ - Pulegone http://dmd.aspetjournals.org/ http://www.springerlink.com/ |
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
clear to yellowish liquid |
|
PURITY |
90.0% min |
|
REFRACTIVE INDEX |
1.485 - 1.490 |
|
SPECIFIC GRAVITY |
0.932 - 0.938 |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
Not regulated |
| HAZARD SYMBOL |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
Combustible liquid and vapor. The toxicological properties of this material have not been fully investigated. May cause eye and skin irritation. May cause respiratory and digestive tract irritation. |
|
GHS |
|
|
SIGNAL WORD |
Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H302 |
|
PRECAUTIONARY STATEMENTS |
|
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
22 |
|
SAFETY PHRASES |
23-24/25 |
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|