|
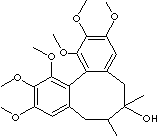
SCHIZANDRIN |
| Synonyms. Gomisins; Magnolia Vine; Wuweizichun A; Wuweizi alcohol A; (+)-Schizandrin; Schisandrin; Schisandrine; Schisandrol A; Schizandrine; 5,6,7,8-Tetrahydro-6,7-dimethyl-1,2,3,10,11,12-hexamethoxydibenzo(a,c) cycloocten-6-ol, stereoisomer; |
|
|
| PRODUCT IDENTIFICATION |
|
|
CAS RN |
7432-28-2 |
|
EINECS RN |
|
|
FORMULA |
C24H32O7 |
|
MOLE WEIGHT |
432.51 |
|
H.S CODE |
2909.49.1000 |
|
SMILES |
c12c3c(cc(OC)c(c3OC)OC)C[C@@H](C)[C@@](Cc1cc(OC)c(c2OC) OC)(C)O |
|
CLASSIFICATION |
Schizandra |
|
EXTRA NOTES |
A dibenzocyclooctadiene lignan present in the fruit of Schisandra chinensis |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES |
|
|
PHYSICAL STATE. |
white to off-white powder |
|
MELTING POINT |
|
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
3.526 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong Oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health hazard: 1, Flammability: 0, Physical hazards: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Drug Information Portal (U.S. National Library of Medicine) - Schizandrin PubChem Compound Summary - Schizandrin Drug Bank - Schizandrin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Schizandrin http://www.ebi.ac.uk/ - Schizandrin http://www.ncbi.nlm.nih.gov/ - Schizandrin http://www.sciencedirect.com/ http://www.sigmaaldrich.com/ |
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
white to off-white powder |
|
ASSAY |
98.0% min |
| OPTICAL ROTATION |
75° ~ 85° |
|
|
| TRANSPORT & REGULATORY INFORMATION |
|
|
UN NO. |
3077 |
| HAZARD SYMBOL |
Class: 9 |
| PACKING GROUP | III |
|
|
| SAFETY INFORMATION |
|
|
HAZARD OVERVIEW |
OSHA Hazards:Harmful by ingestion |
|
GHS |
|
|
SIGNAL WORD |
Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H320-H400 |
|
P STATEMENTS |
P273 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
|
|
SAFETY PHRASES |
|
|
|
| PACKING |
|
|
|
|
| PRICE INFORMATION |
|
|