PRODUCT IDENTIFICATION
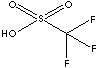
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
Triflic acid, its salts, acid halides, anhydrides and esters are used as a catalyst for the reactions of:
- condensation of alcohols and carboxylic acids
- reaction of aromatic compounds with sulfonyl chlorides
- cracking of alkanes, alkylation of alkenes, isomerisation of alkanes and trans-alkylation of aromatics
- trans-bromination and other Friedel-Crafts reactions.
Substituted trifluoroborates are alternatives to boronic acids in C-C bond forming (rhodium catalyzed) and Suzuki reactions. These salts are stable in air and water. They are excellent leaving groups. They usually don't require adding either additional ligands or base for cross coupling. They do not form cyclic anhydrides which boronic acids do readily. Alkyl triflates are very sensitive to nucleophiles ( SN2 reactions), they must be stored in dried conditions without water.
APPEARANCE
ASSAY
150max
0.1% max
20ppm max
20ppm max
8 (Packing Group: II)
3265
Click