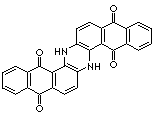
|
VAT BLUE 4 |
| Synonyms. Vat Blue 4; 6,15-Dihydro-5,9,14,18-anthrazinetetrone; C.I. Pigment Blue 60; C.I. 69800; Indanthrene; Anthraquinone Blue; Benzadone Blue RS; Bleu solanthrene; Blue O; Blue anthraquinone pigment; C.I. 1106; Indanthrene Blue; Indanthrone; L-Blau 1; Lake Fast Blue BS; Pigment Blue Anthraquinone; Pigment Blue Anthraquinone V; Vat Blue O; Vat Fast Blue R; Indanthrone blue; Vat Blue RSN; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
81-77-6 |
|
EINECS RN |
201-375-5 |
|
FORMULA |
C28H14N2O4 |
|
MOLE WEIGHT |
442.42 |
|
H.S CODE |
|
|
SMILES |
c12c3c([nH]c4c5c(c(c6ccccc6c5=O)=O)ccc4[nH]3)ccc1c (c1ccc cc1c2=O)=O |
|
CLASSIFICATION |
Anthraquinone |
|
EXTRA NOTES |
|
|
OTHER RN |
11098-09-2, 11129-81-0, 39280-72-3, 50814-33-0, 50926-10-8, 67418-33-1, 88507-35-1, 111116-33-7, 113255-69-9, 128281-88-9, 192588-24-2, 790240-41-4 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
powder |
|
MELTING POINT |
485 C |
|
BOILING POINT |
|
|
DENSITY |
1.6 |
|
SOLUBILITY IN WATER |
3.24E-05 mg/l |
| SOLVENT SOLUBILITY | |
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
7.73 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pH |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 2,Flammability:1, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
Wikipedia Linking - Indanthrone blue Google Scholar Search - Indanthrone blue Drug Information Portal (U.S. National Library of Medicine) - Indanthrone blue PubChem Compound Summary - Solvent Yellow 33 KEGG (Kyoto Encyclopedia of Genes and Genomes) - Indanthrone blue http://www.ebi.ac.uk/ - Indanthrone blue http://www.ncbi.nlm.nih.gov/ - Indanthrone blue http://toxnet.nlm.nih.gov/ http://www.colorantshistory.org/ http://www1.eere.energy.gov/ |
|
|
| SALES SPECIFICATION |
|
|
APPEARANCE |
blue powder or granular |
|
STRENGTH |
100.0% ± 3.0% |
|
Fe |
200ppm max |
|
FASTNESS |
Light (3-4), Wash (4) |
|
MOISTURE |
1.0% max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
Not known |
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
May be harmful if inhaled. May cause respiratory tract irritation. May be harmful if absorbed through skin. May cause skin irritation. May cause eye irritation.May be harmful if swallowed. |
|
GHS |
|
|
SIGNAL WORD |
Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H440-H413 |
|
P STATEMENTS |
P273 |
|
|
| PACKING |
|
|