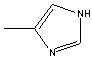
4-METHYLIMIDAZOLE
PRODUCT IDENTIFICATION
822-36-6
H.S. CODE
2933.29
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
colorless to slightly yellow solid
MELTING POINT
46 C
263 C
SOLUBILITY IN WATER
REFRACTIVE INDEX
APPLICATIONS
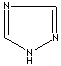
- Triazole: An analog of imidazole.It has three nitrogen atoms and two carbon atoms at nonadjacent positions in the ring system.
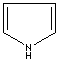
- Pyrrole: An analog of imidazole. It has only one nitrogen atom in the ring system. Pyrrole ring system is involved in coloured products (green pigment, chlorophyll; red, hemoglobin; , blue, indigo) in nature.
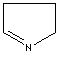
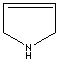
- Pyrroline: A pyrrole in which one of the two solid bonds are hydrogenated.
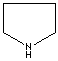
- Pyrrolidine: The saturated tetrahydropyrrole, a part of the structures of amino acids (proline, hydroxyproline and hygrine).
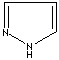
- Pyrazole: 1,2-Diazole (Imidazole isomer). The nitrogen positions are 1 and 2. It is not found in nature
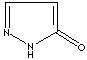
- Pyrazolone: Pyrazole analog with ketone group at 5 positon
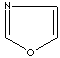
- Oxazole: an analog of imidazole. The nitrogen atom in position 1 is replaced by oxygen.
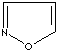
- Isoxazole: an analog of pyrazole. The nitrogen atom at position 1 is replaced by oxygen.
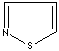
- Isothiazole:an analog of pyrazole. The nitrogen atom at position 1 is replaced by sulfur.
|
|
|
|
|
|
1,2,4-Triazole |
Pyrrole |
1-Pyrroline |
3-Pyrroline |
|
|
|
|
|
|
Pyrrolidine |
Pyrazole |
Pyrazolone |
Oxazole |
|
|
|
|
|
|
Isoxazole |
Isothiazole |
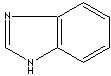
Benzimidazole |
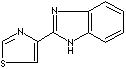
Thiabendazole |
APPEARANCE
colorless to slightly yellow solid
97.0% min
WATER
0.5% max