PRODUCT IDENTIFICATION
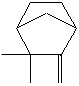
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
FLASH POINT
APPLICATIONS
APPEARANCE
96.0% min
GENERAL DESCRIPTION OF TERPENE
|
Class |
number of isoprene |
Examples |
|
Hemiterpene |
1 (C5H8) |
Found in associated with Alkaloids, Coumarins and Flavonoids. |
|
Monoterpenes |
2 (C10H16) |
Geraniol, Citronellol, Pinene, Nerol, Citral, Camphor, Menthol, Limonene, Thujone |
|
Sesquiterpenes |
3 (C15H24) |
Nerolidol, Farnesol |
|
Diterpenes |
4 (C20H32) |
Phytol, Vitamin A1 |
|
Triterpenes |
6 (C30H48) |
Squalene |
|
Tetraterpenes |
8 (C40H84) |
Carotene (Provitamin A1) |
|
Polyterpenes |
>10 (C5H8)n |
|