PRODUCT IDENTIFICATION
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
150 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Coumarin
PubChem Compound Summary - Coumarin
IPCS INCHEM - Coumarin
Drug Bank - Coumarin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Coumarin
http://www.ebi.ac.uk/chebi/ - Coumarin
http://www.ncbi.nlm.nih.gov/ - Coumarin
Human Metabolome Database - Coumarin
http://doria17-kk.lib.helsinki.fi/
Coumarins
owe their class name to ’coumarou’, the vernacular name of the
tonka bean (Dipteryxodorata Willd., Fabaceae), from which coumarin
itself was isolated in 1820 (BRUNETON, 1999). Coumarins belong to
a group compounds known as the benzopyrones, all of which consist
of a benzene ring joined to a pyrone. Coumarin and the other members
of the coumarin family are benzo-α-pyrones, while the other main
members of the benzopyrone group – the flavonoids –contain the γ-pyrone
group (KEATING and O’KENNEDY, 1997). Coumarins may also be found
in nature in combination with sugars, as glycosides. The coumarins
can be roughly categorised as follows (MURRAY et al., 1982; see
Fig. 1 for coumarins used in this study):·simple – these
are the hydroxylated, alkoxylated and alkylated derivatives of the
parent compound, coumarin, along with their glycosides. ·
furanocoumarins – these compounds consist of a five-membered furan
ring attached to the coumarin nucleus, divided to linear and angular
types with substituents at one or both of the remaining benzenoid
positions. · pyranocoumarins – members of this group are
analogous to the furanocoumarins, but contain a six-membered ring·
coumarins substituted in the pyrone ring.
http://www.leffingwell.com/
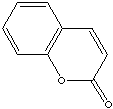
Coumarin
(2H-1-benzopyran-2-one) CAS No 91-64-5, is a crystalline white solid
when seen pure, with a hay-like, sweet aromatic creamy odour with
certain nutty shadings, much used in synthetic form as a fragrance
chemical for perfumes and for fragranced soaps and detergents. Coumarin
has a widespread occurrence in natural products too (see separate
section below), and is a representative of the lactones (where a
lactone is an ester group integrated into a carbon ring system).
http://arxiv.org/
Coumarin
Dyes for Dye-Sensitized Solar Cells – A Long-Range-Corrected Density
Functional Study
http://www.people.vcu.edu/
Biochemical
Mechanism of Action: Coumarins are competitive inhibitors of vitamin
K in the biosynthesis of prothrombin. The coagulation cascade relies
on the conversion of prothrombin to thrombin in a very important
step. However, this conversion depends on the presence of 10 g-carboxyglutamic
acid (GLA) residues in the N-terminus of prothrombin. The multiple
Gla residues form a binding site for Ca+2. Under normal circumstances
10 glutamic acid (Glu) residues of prothrombin are converted to
Gla residues in a post-translational modification. This post-translation
modification is catalyzed by an enzymes vitamin K reductase and
vitamin K epoxide reductase. Vitamin K is a co-factor in this conversion
reaction. Thus it cycles between a reduced form and an epoxide form.
Because of their structural similarity with vitamin K coumarins
are thought to bind the enzymes, vitamin K reductase and vitamin
K epoxide reductase, without facilitating the conversion of Glu
residues of prothrombin to Gla. Thus prothrombin cannot be acted
upon by factor Xa.
Local:
Coumarin (anhydride of o-coumaric acid) is white, crystalline lactone,
obtainable naturally from several plants, such as tonka bean, lavender, sweet
clover grass, strawberries, and cinnamon, or produced synthetically from an
amino acid, phenylalanine. It is the principle of dicumarol which inhibits the
hepatic synthesis of the vitamin K–dependent coagulation factors. Coumarin
derivatives are used widely as anticoagulants (such as
warfarin, -OH group is attached at 4 position) for the treatment of disorders in which there is excessive or
undesirable clotting, such as thrombophlebitis, pulmonary embolism, and certain
cardiac conditions. Coumarin derivatives are also used as rodenticides due
to the property of causing fatal hemorrhaging. Coumarin
has the characteristic odour like that of vanilla beans. It is used for the
preparation of perfumes, soaps, flavourings. The coumarin nucleus (benzo-2-pyrone) is derived cinnamic acid (phenylacrylic
skeleton) in the biosynthesis. Accordingly, the hydroxy group attached to
coumarin structure at 7 position is important in biosynthesis pathway.
Umbelliferone (7-hydroxy coumarin), esculetin (6,7-Dihydroxycoumarin),
scopoletin (7-hydroxy-6-methoxycoumarin) are the widespread coumarins in nature.
Synthetic 7-hydroxy coumarins are used to absorb ultraviolet rays in sunscreen
cosmetics and used in the synthesis of drugs especially anticancer.
|
|
APPEARANCE
99.0% min
MELTING POINT
68 C min
HAZARD OVERVIEW
OSHA Hazards:Target Organ Effect, Toxic by ingestion. Target Organs: Liver, Kidney
GHS
PICTOGRAMS


HAZARD STATEMENTS
H332:
Harmful if inhaled
H312: Harmful in contact with
skin
H302: Harmful if swallowed
H319: Causes serious
eye irritation
H335: May cause respiratory irritation
H351:
Suspected of causing cancer
H315: Causes skin irritation
PRECAUTIONARY STATEMENTS
P280:
Wear protective gloves/protective clothing/eye protection/face
protection
P281: Use personal protective equipment as required
P302
+ P352: IF ON SKIN: Wash with plenty of soap and water
P261:
Avoid breathing dust/fume/gas/mist/vapors/spray
P301+ P312:
IF SWALLOWED: Call a POISON CENTER or doctor/physician if
you feel unwell
P304 + P340: IF INHALED: Remove victim
to fresh air and keep at rest in a position comfortable for breathing
P280:
Wear protective gloves/protective clothing/eye protection/face
protection
P305 + P351 + P338: IF IN EYES: Rinse cautiously
with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing
![]() XN
Harmful
XN
Harmful
RISK PHRASES
20/21/22:
Harmful by inhalation, in contact with skin and if swallowed.
36/37/38:
Irritating to eyes, respiratory system and skin.
40: Limited
evidence of a carcinogenic effect.
SAFETY PHRASES
26:
In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice.
36/37: Wear suitable
protective clothing and gloves.
PRICE INFORMATION