PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
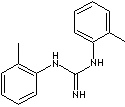
SMILES
CLASSIFICATION
Guanidine, Vulcanization accelerator, Anticonvulsant
PHYSICAL AND CHEMICAL PROPERTIES
white to grayish powder
MELTING POINT
SOLUBILITY IN WATER
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
Di-o-tolylguanidine is used as a secondary rubber accelerator with thiazoles, thiurams, dithiocarbamates and sulfenamides to speed the vulcanization. It is unsuitable for food-contact and medical products. It has similar activity to diphenylguanidine.
http://www.thefreelibrary.com/
.....Activators are chemicals which increase the rate of vulcanization by reacting
first with the accelerators to form rubber soluble complexes. These complexes
then activate the sulfur to effect vulcanization. The most common activators are
combinations of zinc oxide and stearic acid. Other metal oxides have been used
for specific purposes ie. lead, cadmium etc., and other fatty acids used include
lauric and proprionic acids. Soluble zinc salts of fatty acid such as zinc
2-ethyl hexanoate are also available, and these "rubber soluble" activators are
effective in natural rubber to produce low set, low creep compounds used in load
bearing applications. Also, weak amines and amino alcohols have also been used
as activators in combinations with the metal oxides.
Natural rubber
usually contains sufficient fatty acid to solubilize the zinc
salt. However, if the fatty acids are first extracted by acetone, the resultant "clean" natural rubber
exhibits a much lower state of cure. Therefore, to insure consistent cure rate,
fatty acids are usually added for insurance. Synthetic rubbers, especially the
solution polymers, do not contain fatty acids and require their addition to the
cure system.
Sulfenamide accelerators generally require less fatty acid
because they release an amine during the vulcanization process which acts to
solubilize the zinc. Guanidines as similar amine accelerators also serve to both
activate and accelerate vulcanization.
Paris has systematically studied
the effect of stearic acid and zinc oxide on a sulfenamide accelerated, sulfur
cured natural rubber compound (ref. 16). Figure 7 dramatically shows the need
for both the zinc and fatty acid activators.....
APPEARANCE
white to gray powder
INITIAL MELTING POINT
165 C
SIEVE ANALYSIS
0.1% max (+ 150 µm)
OIL CONTENT
1.0 - 2.0%
HEAT LOSS
0.3% max
ASH
0.3% max
OTHER INFORMATION
Hazard Symbols: XN, Risk Phrases: 22, Safety Phrases: 26-36
There are some types of rubber accelerators. They are used in combination with each other in accordance with vulcanizing and/or acid-base conditions. Some examples classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #: 149-30-4)
- Dibenzothiazole disulfide (CAS #: 120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #: 155-04-4)
- Sulphenamide
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #: 95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #: 102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #: 95-31-8)
- Guanidine
- Diphenyl guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #: 10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #: 120-54-7)
- Dithiocarbamate
- Zinc dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl dithiocarbamate (CAS #: 136-23-2)
- Zinc N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
- N-N'-Diphenylthiourea (CAS #: 102-08-9)