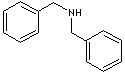
DIBENZYLAMINE
PRODUCT IDENTIFICATION
103-49-1
H.S. CODE
TOXICITY
Oral Rat LD50 : 395 mg/kg
CLASSIFICATION
Incompatibilities
with Other Materials: Strong oxidizing agents, acids, acid chlorides,
acid anhydrides, chloroformates.
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI
Z88.2 requirements or European Standard EN 149 must be followed whenever
workplace conditions warrant respirator use.
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
-26 C
SOLUBILITY IN WATER
2.67 (Octanol-water)
NFPA RATINGS
Health hazard: 3, Fire: 1, Reactivity Hazard: 0
REFRACTIVE INDEX
1.574 - 1.576
143 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Benzylamine
Google Scholar Search - Dibenzylamine
Drug Information Portal (U.S. National Library of Medicine) - Dibenzylamine
PubChem Compound Summary - Dibenzylamine
http://www.ebi.ac.uk/chebi/ - Dibenzylamine
http://www.ncbi.nlm.nih.gov/ - Dibenzylamine
Local:
Amine is a group
of basic organic compounds derived from ammonia (NH3) by replacement
of one (primary amines), two (secondary amines), or three (tertiary amines)
hydrogen atoms by alkyl, aryl groups or organic radicals. Amines, like ammonia,
are weak bases because the unshared electron pair of the nitrogen atom can form
a coordinate bond with a proton. Amines react with acids to give salts and with
acid anhydrides (or ester ) to form amides. They react with halogenoalkanes to
form longer chains.
Many amines are not only bases but also nucleophiles that form a variety of electrophile compounds. They are important intermediates for chemical syntheses due to the basic functionality of the nitrogen atom and electrophilic substitution at nitrogen. Some examples of compounds obtained by reaction of amines are:
- Amides (by reaction with acyl halides or ammonium carboxylate salts)
- N-Alkyl amines (by reaction with halogenoalkanes)
- Isocyanates (by reaction with phosgene)
- Carbamoyl chlorides or Urea derivatives (by reaction with phosgene)
- Alkoxylated amines (by reaction alkylene oxide)
- Quaternary ammonium compounds (by reaction with alkyl halides and dialkyl sulfates)
- N-Alkylcarbamic acids or N,N'- Dialkyl ureas (by reaction with carbon dioxide)
- Urea derivatives (by reaction with isocyanates)
- Schiff bases (by reaction with aldehydes or ketones)
- Aminopropionitriles (by reaction of 1° and 2° amines with acrylonitrile)
- N-Alkylamino acids (by reaction of 1° and 2° amines with monochloroacetic acid or with unsaturated acids)
- Amine oxides (by reaction of 3° amines with hydrogen peroxide)
- Sulfonamide derivatives (by reaction of 1° and 2° amines with benzenesulfonyl chloride)
Aromatic amines such as phenylamine and benzylamine are important for the production of diazonium salts. They dissociate in water (some very weakly). Aromatic amines are much weaker bases than the aliphatics. The term benzyl describes the radical, ion or functional group C6H5CH2-, derived by removing hydrogen atom from methyl group in toluene, while phenyl is the term for the monovalent radical C6H5-, derived by removal of hydrogen from benzene. The common name of phenylamine is aniline. Benzylamine is called aminotoluene or benzenemethanamine in methane nomenclature system. Benzylamine functions in the way as primary aliphatic amines. Benzylamine is a clear liquid boiling at 185 C. It functions in the same way as primary aliphatic amines. Benzylamine and its derivatives are used as chemical intermediate for the manufacture of dyestuffs, pigments, optical brighteners, textile auxiliaries, agrochemicals, amino acids and other organic compounds.
One of the most important aromatic amines is aniline, a primary aromatic amine replacing one hydrogen atom of a benzene molecule with an amino group. It is a pale brown liquid at room temperature; boiling at 184 C, melting at -6 C; slightly soluble in water and freely soluble in ether and alcohol. It causes serious industrial poisoning. The substance may have effects on the blood, resulting in formation of methaemoglobin. Repeated or prolonged exposures may be carcinogenic. Commercial aniline is obtained from nitrobenzene which is prepared from benzene with nitric acid by electrophilic substitution reaction or from chlorobenzene by heating with ammonia in the presence of copper catalyst. It is also obtained as a by-product of coal tar. In commerce the term of aniline oil blue refers to the pure one while aniline oil red indicates a mixture of aniline and toluidines with equimolecular weights. Considerable quantity of aniline is converted into 4,4��-methylenedianiline (MDA) by the condensation reaction of formaldehyde with aniline in the presence of hydrochloric acid. Aniline is the starting material in the dye manufacturing industry. It forms aniline colors when combined with other substances, particularly chlorine or chlorates. Aromatic amines are weaker bases reacting with strong acids to form amides. Anilide is an amide derived from aniline by substitution of an acyl group for the hydrogen of NH2. Acetanilide is from acetic acid and aniline. Acetanilide is an odourless, white flake solid or crystalline powder (pure form); soluble in hot water alcohol, ether, chloroform, acetone, glycerol, and benzene;; melting point 114 C and boiling point 304 C; can undergo self-ignite at 545 C, but is otherwise stable under most conditions. Acetanilide which can be obtained by acetylation of aniline undergoes nitration at low temperature and yields highly the para-nitro products. Acetyl group can then be removed by acid-catalyzed hydrolysis to yield para-nitroaniline. Although the activating affection of the amino group can be reduced, the acetyl derivative remains an ortho/para-orientation and activating substituent. Aniline is converted into sulfanilic acid which is the parent compound of the sulfa drugs. Aniline is also important in the manufacture of rubber-processing chemicals, explosives, plastics, antioxidants and varnishes.
Dibenzylamine find applications predominately in the rubber processing industry. It is used to prepare rubber accelerator for the vulcanization process and reaction-stoppers. Dibenzylamine type accelerators reduce nitrosamine production in the compounding process.
APPEARANCE
colorless to yellow oil liquid
98.0% min
MOISTURE
0.5% max
HAZARD OVERVIEW
OSHA Hazards:Toxic by ingestion, Corrosive. Harmful to aquatic organisms; may cause long-term adverse effects in the aquatic environment. Harmful if swallowed. Causes eye and skin irritation.
GHS
PICTOGRAMS


HAZARD STATEMENTS
H302
Harmful if swallowed.
H313 May be harmful in contact with skin.
H315
Causes skin irritation.
H318 Causes serious eye damage.
PRECAUTIONARY STATEMENTS
P280
Wear protective gloves/ eye protection/ face protection.
P305
+ P351 + P338 IF IN EYES: Rinse cautiously with water for several
minutes. Remove contact lenses, if present and easy to do. Continue
rinsing.
![]() XN
Harmful
XN
Harmful
RISK PHRASES
22:
Harmful if swallowed.
36/38: Irritating to eyes and
skin.
52/53: Harmful to aquatic organisms, may cause long-term
adverse effects in the aquatic environment.
SAFETY PHRASES
26:
In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice.
37/39: Wear suitable
gloves and eye/face protection.
61: Avoid release to the
environment. Refer to special instructions / safety data sheets.