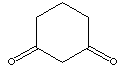
DIHYDRORESORCINOL
PRODUCT IDENTIFICATION
504-02-9
H.S. CODE
2914.29
TOXICITY
1,3-Cyclohexanedione; Cyclohexane-1,3-dione; Hydroresorcinol;
Dihydroresorcinol; Ciclohexano-1,3-diona (Spanish);
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
Beige Crystalline Powder
MELTING POINT
SOLUBILITY IN WATER
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
132 C
GENERAL DESCRIPTION & EXTERNAL LINKS
1,3-Cyclohexanedione is used as an intermediate for pharmaceuticals, herbicides, plant growth regulator and other organic products ( Carvedilol, Ondansetron, Carazolol, Cilansetron, Sulcotrione, Clethodim, Cycloxydim, Mesotrione). Cyclohexanediones can be applicable for the industry of
- Transition-metal complex catalyst chemistry
- Luminescence chemistry and spectrophotometric analysis
- Organic synthesis
- Crystallography and Crystal Chemistry
- Organic low electrical resistance Chemistry
- Colorimetry
Dihydroresorcinol can be used to prepare Wieland-Miescher ketone (a bicyclic di-ketone) a starting material for Taxol total synthesis.
....1,3-Cyclohexanedione (CHD) exists in its enol form in the solid state where it forms hydrogen-bonded chains with an antianti configuration having short (2.56 Å) O-O hydrogen bonding distances.1 This structure is of interest for several reasons including the ordered hydrogen bonding arrangement, an order-disorder phase transition that involves hydrogen bond interchange, and indications derived from examination of crystal structures that CHD is an example of a resonance-assisted, cooperative hydrogen bonding network. This cooperativity arises from the fact that when the enol of CHD forms a hydrogen bond, this polarizes the molecule such that the formation of an additional hydrogen bond with another CHD is more favorable. This may be thought of as due to an enhanced contribution of the ionic resonance form. When crystallized from benzene, this material forms a unique cyclamer structure (CHD6Bz) in which six CHD molecules form a syn-anti hydrogen bonded ring surrounding a central benzene molecule.... (http://webster.ncnr.nist.gov/)
APPEARANCE
beige crystalline powder
96.0% min
MELTING POINT
101 C
WATER
0.1% max