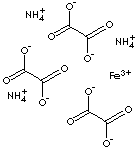
| FERRIC AMMONIUM OXALATE TRIHYDRATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO | 2944-67-4,
14221-47-7, 55488-87-4 (anhydrous) 13268-42-3 (trihydrate) |
|
| EINECS | 220-952-2, 238-090-0, 259-669-4 | |
| FORMULA | (NH4)3Fe(C2O4)3· 3H2O | |
| MOL WT. | 428.06 | |
|
H.S. CODE |
2917.11.0000 | |
|
TOXICITY |
||
| SYNONYMS | Triammonium Iron(3+) trioxalate; Triammoniumtrioxalatoferrat; | |
| Ammonium Iron (III) oxalate; Triammonium eisen(3+) trioxalat; Oxalsäure Ammoniumeisensalz (German); Trioxalato de triamonio y hierro(3+); trioxalatoferrato de triamonio; ácido oxálico, sal de amonio y hierro (Spanish); Trioxalate de triammonium et de fer(3+); Trioxalatoferrate de triammonium; Acide oxalique, sel d'ammonium et de fer (French); Iron (III) ammonium oxalate trihydrate; Potassium ferrioxalate ammonium salt; | ||
| SMILES | OC(C([O-])=O)=O.OC(C([O-])=O)=O.OC(C([O-])=O)=O.N.N.N. [Fe+3] | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
green crystals (vary in color according to the amount of iron) | |
| MELTING POINT | Decomposes | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | 1.78 | |
| SOLUBILITY IN WATER | Soluble | |
| SOLVENT SOLUBILITY | Insoluble in alcohol | |
| pH | ||
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 2; Flammability: 1; Reactivity: 0 | |
| AUTOIGNITION |
| |
| REFRACTIVE INDEX |
| |
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. Hygroscopic. Light sensitive. | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION |
||
|
PubChem Compound Summary - Ferric ammonium oxalate Local: |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
green crystals | |
| ASSAY |
98.0% min | |
|
Fe CONTENT |
12.6 - 13.3% | |
|
SO4 |
0.03% max | |
|
As |
5ppm max | |
|
HEAVY METALS |
10ppm max | |
|
WATER INSOLUBLES |
0.5% max | |
| TRANSPORTATION | ||
| PACKING | 25Kgs in bag | |
| HAZARD CLASS | ||
| UN NO. |
Not regulated | |
| SAFETY INFORMATION | ||
|
HAZARD OVERVIEW |
Hygroscopic. Light sensitive. Target Organs: Respiratory system, eyes, skin. |
|
|
GHS |
|
|
| SIGNAL WORD | Danger | |
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H318 Causes serious eye damage |
|
|
PRECAUTIONARY STATEMENTS |
P301 + P330 + P331 IF SWALLOWED: rinse mouth. Do NOT induce
vomiting |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
34 Causes burns |
|
|
SAFETY PHRASES |
36/37/39 Wear suitable protective clothing, gloves and
eye/face protection. |
|
| PRICE INFORMATION | ||
|
|
||