PRODUCT IDENTIFICATION
204-712-4
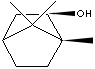
SMILES
CLASSIFICATION
Flavouring Substance, Alcohol, Monoterpene
PHYSICAL AND CHEMICAL PROPERTIES
212 - 214 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
Wikipedia Linking:http://en.wikipedia.org/wiki/Isoborneol
http://chestofbooks.com/
This alcohol which results together with borneol upon reduction of camphor, and
which, according to the latest views, is regarded as stereoisomeric with
borneol, can also be obtained upon hydration of camphene.4) According to Bertram
and Walbaum it melts at 212° in the sealed tube. Its boiling point cannot be
ascertained since the alcohol begins to sublime before the boiling temperature
has been reached. Its phenylurethane melts at 138 to 139° and regenerates
Isoborneol when treated with alcoholic potassa. When heated with dehydrating
agents camphene results. The reactions by means of which isoborneol can be
distinguished from borneol have already been discussed under the latter.
http://encyclopedia2.thefreedictionary.com
bornyl alcohol, a secondary alcohol of the bicyclic terpene group. Borneol
has an endo configuration; its isomer, called isoborneol, has an exo
configuration. [The (CH3)2C group is located outside the
plane of the ring.] Borneol and isoborneol contain an asymmetric carbon atom (denoted by an
asterisk), and therefore each of them exists in two optically active forms and
one inactive racemic form (-, +, and ±). Borneo camphor, (+)-borneol, is
contained in the secretions of the tree Dryobanalops camphora, which
grows on the islands of Borneo and Sumatra, as well as in rosemary, lavender,
and camphor oils. The acetate form, (-)-borneol, is contained in the
coniferous needle of the Siberian pine. Both (+)-borneol and (-)-borneol have a
melting point Tm = 208.5° C, boiling point Tb = 212° C,
and angle of rotation of the plane of polarization [α]D20
= ±37.9°; and both sublimate. The racemate (±)-borneol melts at 210.3° C. Both
(-)-borneol and (±)-borneol are components of valerian oil....
APPEARANCE
MOISTURE
1.0% max
FEMA No.: 2158