| CAS
NO. |
101-25-7 |
|
| EINECS
NO. |
202-928-3 |
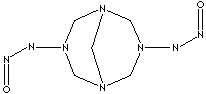
| FORMULA |
C5H10N6O2 |
| MOL
WT. |
186.17 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
3,7-Dinitroso-1,3,5,7-tetraazabicyclo[3.3.1]nonane; |
|
3,7-Di-N-nitrosopentamethylenetetramine;
DPT; 1,5-Endo-Methylene-3,7-dinitroso- 1,3,5,7-tetraazacyclooctane;
1,5-Methylene-3,7-dinitroso-1,3,5,7- tetraazacyclooctane; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
light
yellow powder |
|
MELTING
POINT
|
|
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
1.45 |
|
SOLUBILITY
IN WATER
|
|
| pH |
6.5
- 7.5 |
| VAPOR
DENSITY |
|
| AUTOIGNITION |
|
|
REFRACTIVE
INDEX
|
|
| NFPA
RATINGS |
|
| FLASH
POINT |
|
| STABILITY |
Stable
but decomposes if heated |
|
GENERAL
DESCRIPTION
& APPLICATIONS
|
| Blowing agents, also called Foaming agents, can
be classified as Physical and Chemical Blowing Agents. Physical
blowing agents are not under chemical changes during processing.
Physical blowing agents are in forms of liquid or compressed
gas which will transfer state into gases or low boiling
liquid during processing causing resins into cellular structure.
Chemical Blowing Agents are mainly solid form of hydrazine
derivatives include Azodicarbonamide;
p,p'-Oxybis(benzenesulfonyl hydrazide); 5-Phenyltetrazole;
p-Toluene sulfonyl semicarbazide;
Trihydrazine Triazine; They commonly release gases such
as nitrogen, carbon dioxide, carbon monoxide or ammonia.
But ammonia is not desirable as it effects on degrade of
resins. There are nonazo blowing agents releasing carbon
dioxide such as sodium borohydride.
Blowing
Agent Class
- Azo
Compounds
- Azodicarbonamide
(CAS RN: 123-77-3)
- Hydrazine
Compounds
- p-Toluenesulfonylhydrazide
(CAS RN: 1576-35-8)
- p,p'-Oxybis
(Benzenesulfonylhydrazide) (CAS RN: 80-51-3)
- Benzenesulfonyl Hydrazide
(CAS RN: 80-17-1)
- p-Toluenesulfonyl acetone hydrazone
- Carbazides
- p-Toluenesulfonylsemicarbazide
(CAS RN: 10396-10-18)
- p,p'-Oxybis
(Benzenesulfonylsemicarbazide)
- Tetrazoles
- 5-Phenyltetrazole
(CAS RN: 18039-42-4)
- Nitroso
Compounds
- N,N’-Dinitroso-pentamethylenetetramine
(CAS RN: 101-25-7)
- Carbonates
- Sodium
Bicarbonate (CAS RN: 144-55-8)
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
light
yellow powder |
|
ASSAY
|
97.0%
min
|
|
ASH
|
0.2%
max
|
| DECOMPOSITION
TEMP |
175
- 185
C
|
| GAS
YIELD |
275
- 285 (ml/g)
|
| PARTICLE
SIZE |
14
- 17 µm
|
|
WATER
|
0.3%
max
|
| TRANSPORTATION |
| PACKING |
25kgs
in Bag, 40kgs in iron drum |
| HAZARD
CLASS |
4.1
(Packing group : III) |
| UN
NO. |
3242 |
| GENERAL
DESCRIPTION
OF NITROSO- |
|
Nitroso
is the prefix for NO-
radical with trivalent nitrogen also known as hydroximino;
oximido. Substances in which this group is attached
to an oxygen atom are called nitrites (esters of nitrous
acid); those in which the nitroso group is attached
to a metal ion are called nitrosyls. Nitroso (-N=O)
groups and azo (-N=N-) groups impart colour to the compound
as these groups absorb light of characteristic wavelengths.
Nitroso groups are strongly electron withdrawing and more similar to the C=O
than the nirto group. Nitroso compound tends to dimerise. There is polarisation
of the N=O bond and so behaves as a weak C=O. It undergoes addition of
nucleophiles and condensation with primary amines and the anions of active
methylene compounds. Nitroso groups are useful in addition and condensation
reactions, stable nitroxide radical preparations, and isomerization to oxime.
Oxidation undergoes readily with peracids, this is not available with nitro
groups. Nitroso compounds are useful intermediates for the synthesis of target
materials. Certain niroso derivatives are used as vulcanization accelerator by direct
reacting with peroxy-free radical to tie up free radicals and adhesion
promoter between metal and rubber. |
