| CAS
NO. |
95-31-8 |
|
| EINECS NO. |
202-409-1 |
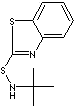
| FORMULA |
C11H14N2S2 |
| MOL
WT. |
238.37 |
| H.S.
CODE |
2934.20 |
| TOXICITY |
Mouse
LD50 (intravenous): 180mg/kg |
| SYNONYMS |
N-tert-Butyl-2-Benzothiazolesulfenamide;
TBBS; |
| 2-(tert-Butylaminothio)benzothiazole;
N-(1,1-Dimethylethyl)-2-benzothiazolesulfenamide; Benzothiazolyl-2-tert-butylsulfenamide; N-(1,1-Dimethylethyl)benzothiazolesulfenamide; Santocure NS vulcanization accelerator; Vanax
NS; Vulkacit NZ; Other RN: 40860-78-4; 172451-95-5; 179095-45-5 |
| SMILES |
c12c(sc(n1)SNC(C)(C)C)cccc2
|
|
CLASSIFICATION
|
Vulcanization accelerator
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
off
white to tan powder
|
| MELTING POINT |
105
C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| AUTOIGNITION |
|
| pH |
|
| VAPOR DENSITY |
|
| NFPA RATINGS |
Health: 1; Flammability: 0; Reactivity: 0 |
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions |
|
APPLICATIONS
|
|
Sulfur combines with nearly all elements. Sulfur forms ring and chain structures
as it is the second only to carbon in exhibiting catenation. The 8-membered ring and
shorter chain structure of sulfur molecule is important in vulcanization
process which individual polymers are linked to other polymer molecules by
atomic bridges. This process produces thermoset materials which are cross-linked
and irreversible substances. The term thermoplastic is for high molecular weight
polymers which can undergo melting-freezing cycle. Thermosets are not melted and
re-molded on heating after cured. The split of sulfur 8-membered ring structure into shorter chains provides rubber vulcanization process. The split are
liked with cure sites (some of the solid bonds in the molecule) on rubber
molecules, resulting in forming sulfur bridges typically between 2 and 10 atoms
long. Vulcanization makes rubber harder, more durable and more resistant to
heating, aging and chemical attacks. The number of sulfur atoms in the sulfur bridges varies physical properties of
the end products. Short bridges containing one or two sulfur atoms offer heat
resistance and long bridges offer flexible property. Vulcanization can also be accomplished with
certain peroxides, gamma radiation, and several other organic compounds. The
principal classes of peroxide cross-linking agents are dialkyl and diaralkyl
peroxides, peroxyketals and peroxyesters. Other vulcanizing agents include amine
compounds for the cross-linking of fluorocarbon rubbers, metal oxides for
chlorine-containing rubbers (notably zinc oxide for chloroprene rubber) and
phenol-formaldehyde resins for the production of heat-resistant butyl rubber
vulcanizates. Accelerator, in the rubber industry, is added with a curing agent
to speed the vulcanization. Accelerators contain sulfur and nitrogen like derivatives of benzothiazole and thiocarbanilides.
The popular accelerators are
sulfenamides (as a delayed-action accelerators), thiazoles, thiuram sulfides,
dithocarbamates and guanidines.
There are some types of rubber accelerators. They are used in combination with each other in
accordance with vulcanizing and/or acid-base conditions. Some examples
classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #:
149-30-4)
- Dibenzothiazole disulfide (CAS #:
120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #:
155-04-4)
- Sulphenamide
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #:
95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #:
102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #:
95-31-8)
- Guanidine
- Diphenyl
guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl
thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl
thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl
thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl
thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #:
10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #:
120-54-7)
- Dithiocarbamate
- Zinc
dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl
dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl
dithiocarbamate (CAS #: 136-23-2)
- Zinc
N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc
dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper
dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene
thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
- N-N'-Diphenylthiourea (CAS #:
102-08-9)
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
off
white to tan dust suppressed powder
|
| ASSAY |
95.0% min
|
|
MELTING
POINT
|
105
C (Initial), 107 - 112 C (final)
|
|
MOISTURE
|
0.4%
max
|
|
ASH
|
0.4%
max
|
|
INSOLUBLES
|
0.5%
max (in methanol)
|
|
PARTICLE
SIZE
|
0.1%
max (+150 µm), 0.5% max (+65 µm)
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| GENERAL DESCRIPTION & THIAZOLE |
Thiazole is a
heterocyclic organic compound that has a five-member ring molecular structure,
C3H3NS, containing three
carbon atoms, one sulphur atom, and one nitrogen atom. It is a clear to
yellowish liquid with a pyridine like odor. It is soluble in alcohol and ether
and slightly soluble in water. It is parent material for numerous of chemical
compounds including sulfur drugs, biocides, fungicides, dyes, chemcal reaction
accelerators. Thiazole dyes contain the color radicals of =C=N- and -S-C= which
decide colors to a compound . Azo dyes contain -N=N- radical. Thiazole dyes are
useful in dying cotton. Benzothiazole is a thiazole fused to a benzene ring.
Benzothiazole derivatives are important in the industry of dyes, photographic chemicals, sulfa
drugs and rubber. Thiabendazole, a
substituted derivative of benzimidazole, is used as broad-spectrum anthelmintic
and presevative. Some thiazole derivatives, belong to a class of cyclic sulfur
organo products containing sulfur atom (S) and often oxygen (O), nitrogen (N),
hydrogen (H), as well as other elements, can find application for making
biological activitive agents such as antiviral, antibacterial, antifungal ,
antituberculous, antbody and antifungal agents. Thiazole and its derivatives
are also used as thiol flavouring substances.
|
