PRODUCT IDENTIFICATION
CLASSIFICATION
OXIMES /
PHYSICAL AND CHEMICAL PROPERTIES
GENERAL DESCRIPTION AND APPLICATIONS
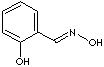
Oxime is any compound with the general formula R\R'/C=N-OH, where R and R' are hydrogen atoms or organic groups. Oximes are condensation products of hydroxylamines with aldehydes (forming aldoxime), ketones (forming ketoxime), or quonone. Aldoximes exist only as a syn isomer. But benzaldoxime (aromatic aldoximes) exist in syn- and anti isomers: the syn form melts at 34C, antiisomeric form at 130 C; both forms are soluble in ethanol and ether.
There are two geometrical isomer: syn and anti isomer (the term syn-anti isomerism is for stereoisomers by other atoms' unsaturated bond rather than carbon). Two isomers have very different properties. The conversion of oximes into corresponding amides, known as Beckmann rearrangement (usually using sulphuric acid as a catalyst), is used to make synthetic fiber monomers. Cyclhexanone oxime is converted into its isomer epsilon-caprolactam which is the raw material to make nylon-6. The amides obtained by Beckmann rearrangement can be converted into amines by hydrolysis, which are useful in the manufacture of dyes, plastics, synthetic fibres, and pharmaceuticals.Oximes are used as a peel-preventing additive in paints and lacquers. It acts as an antioxidant against oxidative drying materials which forms sticky skin with air oxygen. The another effect of anti-skinning offers drying time delay which can be used in formulating paints.
Oximes are used as chemical building block for the synthesis of agrochemicals and pharmaceuticals. In medicine application, Oxime structure is effective in cholinesterase reactivators to treat the poisoning by organophosphates. Example of these drugs are pralidoxime, obidoximine, methoxime, asoxime, and trimedoxime. Oxime moiety is found in some cephalosporin antibiotics. Diacetyl monoxime is used as an inhibitor of ATP-sensitive potassium ion channels.
Diacetyl (dimethylglyoxal) reacts with hydroxylamine to produce diacetyldioxime (dimethylglyoxime). The characteristic property of oxime is the scavenging free radical and oxygen. Diacetyl oxime is used as a chelating agent. An application example is the spectrophotometric determination of Co(II), Fe(II), Ni(II), Pd(II) and Re(VII)1. Dimethylglyoxime reacts with a nickel salt in a green solution to generate a red colored insoluble tetradentate coordinate complex of nickel. It is used as a reagent for the colorimetric determination of urea and ureido compounds.
Oxime is used as a ligand in transition-metal complex catalyst chemistry. Oxime acts as an antioxidant, radical scavenger which find applications in textile, plastic, paint, detergent, and rubber industry.
APPEARANCE
99.0% min
0.5% max