| tert-BUTYL HYDROPEROXIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO | 75-91-2 |
|
| EINECS NO. | 200-915-7 | |
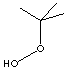
| FORMULA | (CH3)3COOH | |
| MOL WT. | 90.14 | |
|
H.S. CODE |
||
|
TOXICITY |
Oral rat LD50: > 90 ml/kg | |
| SYNONYMS | 1,1-Dimethylethyl Hydroperoxide;TBHP; T hydro; | |
| 2-Hydroperoxy-2-Methylpropane; Dimethylethyl hydroperoxide; Butyl hydroperoxide; tertiary-Butyl hydroperoxide; Trigonox; | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES (70% AQ. SOL.) |
||
| PHYSICAL STATE | clear liquid | |
|
MELTING POINT |
< -1 C | |
| BOILING POINT | 89 C | |
| SPECIFIC GRAVITY | 0.93 - 0.94 | |
|
SOLUBILITY IN WATER |
soluble | |
| pH | weak acid (Aq sol.) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
238 C | |
|
NFPA RATINGS |
Health: 3; Flammability: 2; Reactivity: 2 | |
| REFRACTIVE INDEX |
||
| FLASH POINT |
43 C | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Peroxide: Compound containing the peroxy group (-O-O-), chainlike structure,
containing two oxygen atoms, each of which is bonded to the other and to a
radical or some element. It is considered that hydrogen peroxide is the starting
material to prepare organic and inorganic peroxides commercially. Hydrogen
Peroxide H2O2, is a powerful oxidizing agent. The most valuable property of
hydrogen peroxide is that it breaks down into water and oxygen and therefore
does not form any persistent, toxic residual compounds. It is used in the
processes of epoxidation, oxidation, hydroxylation and reduction. Its oxidizing
properties are used in the bleachings and deodorizing for textile, hair and in
paper manufacture. It is also used medicinally as an antiseptic. Its
application involves the production of chemicals like perhydrates as well as
organic peroxides in which some organic (or inorganic) substituents have
replaced one or both hydrogens. Some metals form peroxides in air sodium, barium
or zinc. Metal peroxide releases oxygen slowly in contact with atmospheric
moisture and used to as disinfectants in cosmetics, detergents, toothpaste and
pharmaceuticals. They can be used in the bleachings and deodorizing and a oxygen
release source in agricultural application to generate contaminated soils and
lakes. Organic Peroxides are powerful oxidizing agents releasing oxygen. They
are widely used as initiators,catalysts and crosslinking agent for the
polymerization process in the plastics manufacturing industry and as chemical
intermediates, bleaching agents, drying and cleaning agents. They are also used
as antiseptics, disinfectants and germicides medically for cosmetics,
detergents, toothpaste and pharmaceuticals. Organic peroxides are classified
in peroxydicarbonates, peroxyketals, peroxyesters, ketone
peroxides, hydroperoxides, dialkyl peroxides, diacyl peroxides by HMIS.
Tert-Butyl Hydroperoxide has a high thermal stability compared to other organic peroxides and is one of the safest organic peroxides. It decomposes rapidly, causing fire and explosion hazard, on heating and under influence of light. It reacts violently with incompatible substances or ignition sources (acids, bases, reducing agents, and heavy metals). It should be stored in a dry and refrigerated (< 27C recommended or 38 C max) area and to keep away from reducing agents and incompatible substances. It is sold commonly as a dilution containing 69-70 w/w% in water. It is soluble in the most solvents hydrocarbon solvents as well as in water. This solubility makes it a versatile oxidation agent for selective aqueous or non-aqueous oxidations. It is used as a free radical initiator over a wide temperature range under appropriate redox initiation. TBHP is one of the general cationic or curing agent for emulsion and suspension polymerization of ethylene, vinylacetate, acrylates. PVC, unsaturated polyesters. The one of main application of TBHP is the source of tertiary-butyl derivatives including peresters, perketals, and alkyl peroxides. TBHP undergoes epoxidation reaction of alpha-olefins in the presence of various choice of transition metal catalysts. The choice of metal catalysts is important to control selectively reactivity and to minimize side reactions and to determine chemo-, regio-, and stereoselectivity. It is an useful selective oxidation source in presence of transition metal catalysts. It oxidizes sulfur compounds to soluble derivatives to be removed in petroleum refinery. It is used as an additive in lubricants to reduce the wear of metal the surfaces. It is also used in bleaching, waste treatment and disinfectant. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
| CONTENT | 69.0 - 70.0% | |
|
ACTIVE OXYGEN |
12.0% min | |
| SPECIFIC GRAVITY |
0.93 - 0.94 | |
|
WATER |
Balance | |
| TRANSPORTATION | ||
| PACKING | 170kgs in drum | |
| HAZARD CLASS | 5.2 (Packing Group:II) | |
| UN NO. | 3109 | |
| OTHER INFORMATION | ||
| Hazard Symbols: O C, Risk Phrases: 7/10/20/21/22/34/52/53, Safety Phrases: 3/7/14A/16/17/36/37/39/45 | ||