| CAS
NO. |
77-93-0 |
|
| EINECS
NO. |
201-070-7 |
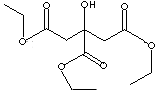
| FORMULA |
HOC(CO2C2H5)(CH2CO2C2H5)2 |
| MOL
WT. |
276.29 |
| H.S.
CODE |
|
| TOXICITY |
Orl
rat LD50: 5900 mg/kg |
| SYNONYMS |
Citric Acid, Triethyl Ester;
TEC; |
|
Ethyl citrate; Triaethylcitrat
(German); Triethylester Kyseliny Citronove (Czech);
2-hydroxy-1,2,3-propanetricarboxylic
Acid, Triethyl ester; Citroflex 2; |
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
clear
liquid
|
| MELTING POINT |
-45
C |
| BOILING
POINT |
294
C |
| SPECIFIC GRAVITY |
1.137 |
| SOLUBILITY
IN WATER |
|
| AUTOIGNITION |
|
| pH |
|
| VAPOR DENSITY |
5.56 |
| NFPA RATINGS |
Health: 0 Flammability: 1 Reactivity: 0 |
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
155
C
|
| STABILITY |
Stable under ordinary conditions |
|
APPLICATIONS
|
|
Triethyl Citrate
is used as a high boiling solvent and plasticizer for vinyl resins,
cellulose
acetates. It is a plasticizer permitted in the field of food additive, food contact material,
medical,
and pharmaceutical. It
is used as a plasticiser and humectant for cigarette
filters.
It is widely used in cosmetics , lacquers and as a fragrance carrier. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
clear
liquid
|
|
ESTER CONTENT
|
99.0%
max
|
|
WATER
|
0.25%
max
|
|
ACID
VALUE
|
0.2
max (mg KOH/g)
|
|
Specific Gravity |
1.135 - 1.139 |
|
Refractive Index |
1.440 - 1.442 |
|
Heavy Metals |
10ppm
max
|
|
COLOR,
APHA
|
50
max
|
| TRANSPORTATION |
| PACKING |
225kgs
in drum |
| HAZARD CLASS |
Not
regulated |
| UN
NO. |
|
| GENERAL
DESCRIPTION OF CITRIC ACID
|
|
Citric Acid (2-Hydroxy-1,2,3-propanetricarboxylic acid, in IUPAC naming) is a
colourless crystalline organic compound belong to carboxylic acid family. It
exists in all plants (especially in lemons and limes) and in many animal tissues
and fluids. In biochemistry, it is involved in important metabolism of almost
all living things; the Krebs cycle (also called citric acid cycle or
tricarboxylic acid cycle), a part of the process by which animals convert food
to energy. Citric acid works as a preservative ( or as an antioxidant) and
cleaning agent in nature. It is commercially obtained by fermentation process of
glucose with the aid of the mold Aspergillus niger and can be obtained
synthetically from acetone or glycerol. It can be used as an sour taste enhancer
in foods and soft drinks. The three carboxy groups lose protons in solution;
resulting in the excellent pH control as a buffer in acidic solutions. It is
used as a flavouring, stabilizing agent and acidulant (to control acidity) in
food industry, in metal-cleaning compositions as it chelates metals. Citric acid
is available in forms of anhydrous primarily and in monohydrate, the
crystallized form from water. The hydrated form will be converted to the
anhydrous form above 74 C. Citrate is a salt or ester of citric acid. Citrates
are formed by replacing the acidic one, two, or all three of the carboxylic
hydrogens in citric acid by metals or organic radicals to produce an extensive
series of salts, esters, and mixed (solid) salts. Cirrates are used in food,
cosmetics, pharmaceutical and medicine industries as well as in plastic
industry; nutrient or food additives having functions of acidity regulator,
sequestering and stabilizing agent, antioxidants synergist, firming agent;
anticoagulant for stored whole blood and red cells and also for blood specimens
as citrates chelate metal ions and saline cathartics, effervescent medicines;
high boiling solvent, plasticizer and resin for food contact plastics. |
