PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
MELTING POINT
97 - 105 C
SOLUBILITY IN WATER
Insoluble
SOLVENT SOLUBILITY
Soluble in toluene. acetone, ether, ethanol
REFRACTIVE INDEX
GENERAL DESCRIPTION & EXTERNAL LINKS
Wikipedia Linking: http://en.wikipedia.org/wiki/Accelerant
http://www.springerlink.com/
Rubber
accelerators are now being fabricated in finely dispersed
powder form, which improves the distribution of the
compounds in rubber mixes. But the dispersion of the
accelerators causes lumping and caking, and this hinders
process mechanization and automatic control in the preparation
and processing of rubber mixes. Granulation of rubber
cure accelerators results in material which flows freely
and is easily transported, but the accelerator should
retain its finely dispersed structure. Granules satisfying
this requirements can be produced on extruders by taking
advantage of the tendency of aqueous pastes of rubber
vulcanizing accelerators to plasticize when subjected
to intense mixing and grinding.
http://doc.utwente.nl/
The effects of N-cyclopentamethylene
thiocarbamyl-N-(cyclohexyl,thiocyclohexyl)sulfenamide (CPCTS) and
N-oxydiethylene thiocarbamyl-N-(cyclohexyl,thiocyclohexyl)sulfenamide (ODCTS) as
cure modifiers on the vulcanization of natural rubber (NR) containing
benzothiazole accelerators were studied. CPCTS and ODCTS were used separately
with 2-mercaptobenzothiazole and N-oxydiethylene benzothiazole sulfenamide to
determine their effects on these accelerators. CPCTS and ODCTS retarded the
accelerators. Enhanced activity was found with respect to the torque, scorch,
modulus, and tensile strength and was believed to be due to the formation of
different intermediate components during the early stage of the vulcanization of
NR. Reactions likely responsible for acceleration and retardation were explored.
CPCTS and ODCTS provided better cure retardation than N-cyclohexyl
thiophthalimide. The chemical characterization of the NR vulcanizates correlated
with the physical properties obtained at 140°C. Stocks containing CPCTS or ODCTS
produced mostly monosulfide linkages, which were affected when N-cyclohexyl
thiophthalimide was used. This provided information concerning the
heat-resistance properties of the vulcanizates
SALES SPECIFICATION
APPEARANCE
ASSAY (TITRATION)
94.0% min
MELTING POINT
96 C (Initial) , 100 - 105 C (Final)
SIEVE ANALYSIS
0.5% max (+ 63 µm), 0.1% max (+ 150 µm)
INSOLUBLE
0.5% max ( in methanol)
ASH
0.3% max
OIL CONTENT
2.0% max (According to Buyer's request)
MOISTURE
0.5% max
Hazard Symbols: XI N, Risk Phrases: 31-43-50/53, Safety Phrases: 36/37-60-61
There are some types of rubber accelerators. They are used in combination with each other in accordance with vulcanizing and/or acid-base conditions. Some examples classified by chemical structure are as below;
- Thiazole
- 2-Mercaptobenzothiazole (CAS #: 149-30-4)
- Dibenzothiazole disulfide (CAS #: 120-78-5)
- 2-Mercaptobenzothiazole Zinc salt (CAS #: 155-04-4)
- Sulphenamide
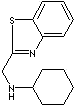
- N-Cyclohexyl-2-benzothiazole sulfenamide (CAS #: 95-33-0)
- N-Oxydienthylene-2-benzothiazole sulfenamide (CAS #: 102-77-2)
- N-tert-butyl-2-benzothiazyl sulfenamide (CAS #: 95-31-8)
- Guanidine
- Diphenyl guanidine (CAS #: 102-06-7)
- Di-o-tolylguanidine (CAS #: 97-39-2)
- Thiuram
- Tetramethyl thiuram disulfide (CAS #: 137-26-8)
- Tetraethyl thiuram disulfide (CAS #: 97-77-8)
- Tetramethyl thiuram monosulfide (CAS #: 97-74-5)
- Isobutyl thiuram disulfide (CAS #: 3064-73-1)
- Tetrabenzylthiuram disulfide (CAS #: 10591-85-2)
- Dipentamethylene thiuramtetrasulfide (CAS #: 120-54-7)
- Dithiocarbamate
- Zinc dimethyl dithiocarbamate (CAS #: 137-30-4)
- Zinc diethyl dithiocarbamate (CAS #: 14324-55-1)
- Zinc dibutyl dithiocarbamate (CAS #: 136-23-2)
- Zinc N-ethyl-dithiocarbamate (CAS #: 14634-93-6)
- Zinc dibenzyl dithiocarbamate (CAS #: 14726-36-4)
- Copper dimethyl dithiocarbamate (CAS #: 137-29-1)
- Thiourea
- Ethylene thiourea (CAS #: 96-45-7)
- N,N'-Diethylthiourea (CAS #: 105-55-5)
- N-N'-Diphenylthiourea (CAS #: 102-08-9)
PRICE INFORMATION