| ANTHRONE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 90-44-8 |
|
| EINECS NO. | 201-994-0 | |
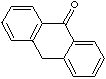
| FORMULA | C14H10O | |
| MOL WT. | 194.23 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | 9,10-Dihydro-9-oxoanthracene; Carbothrone; | |
| 9(10H)-Anthracenone; 9,10-Dihydro-9-oxoanthracene; 9-Oxoanthracene; hracene, | ||
| DERIVATION | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White to yellow crystals | |
| MELTING POINT | 150 - 155 C | |
| BOILING POINT | 720 C | |
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Insoluble | |
| SOLVENT SOLUBILITY |
||
| AUTOIGNITION |
| |
| pH |
| |
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 1 Flammability: 1 Reactivity: 0 | |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. Light sensitive. | |
|
APPLICATIONS |
||
|
Anthracene is a tricyclic aromatic hydrocarbonn derived from coal tar; melts at 218°C,boils at 354°C, insoluble in water but is soluble in most organic solvents such as carbon disulfide, alcohols, benzene, chloroform, and hydronaphthalenes. Its molecular structure consists of three benzenelike rings joined side by side (the general formula CnH2n-18) and its oxidation yields anthraquinone, the parent substance of a large class of dyes and pigments. Anthracene is a basic substance for production of anthraquinone, dyes, pigments, insecticides, wood preservatives and coating materials. Anthracene is a nucleus for polymer soluble pigments. Anthracene forms reversible photodimer through the 9-,10- positions in response to light and provides photochromic applications. Anthracene family compounds are base materials for colorings. They have useful functions such as light and temperature sensitivity, heat resistance, conductivity, emittability, and corrosion resistance. Due to pi-electron cloud overlaps, anthracene exhibits semiconductor property. Organic semiconductors have some merits of self radiation, flexibility, light weight, easy fabrication, and low cost. They have been investigated as organic electroluminescence materials for the applications in organic solar cells, biosensitizers and display devices such as OLED(Organic Light Emiting Diode), OTFT(Organic Thin Film Transistor), Wearable Display, and e-paper. Some examples of organic electroluminescence materials are:
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White to yellow crystals | |
| ASSAY |
98.0% min | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 24/25 | ||